Taxonomy and Distribution of the Argentine Ant, Linepithema Humile (Hymenoptera: Formicidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Winged Ants (Hymenoptera: Formicidae) Presence in Twigs on the Leaf Litter of Atlantic Forest
Biota Neotropica 19(3): e20180694, 2019 www.scielo.br/bn ISSN 1676-0611 (online edition) Inventory Winged ants (Hymenoptera: Formicidae) presence in twigs on the leaf litter of Atlantic Forest Tae Tanaami Fernandes1 , Rogério R. Silva2, Débora Rodrigues de Souza-Campana2, Otávio Guilherme Morais da Silva2 & Maria Santina de Castro Morini1* 1Universidade de Mogi das Cruzes, Laboratório de Mirmecologia do Alto Tietê, Rua Dr. Cândido Xavier de Almeida e Souza, 200, CEP 08780-911, Mogi das Cruzes, SP, Brasil 2Museu Paraense Emílio Goeldi, Coordenação de Ciências da Terra e Ecologia, Avenida Perimetral, 1901, Terra Firme, CEP 66077-830, Belém, PA, Brasil *Corresponding author: Maria Santina de Castro Morini, e-mail: [email protected] FERNANDES, T. T., SILVA, R. R., SOUZA-CAMPANA, D. R., SILVA, O. G. M., MORINI, M. S. C. Winged ants (Hymenoptera: Formicidae) presence in twigs on the leaf litter of Atlantic Forest. Biota Neotropica 19(3): e20180694. http://dx.doi.org/10.1590/1676-0611-BN-2018-0694 Abstract: In the leaf litter, ants have various nesting resources available, such as live or dead trunks, twigs, leaves, fruits and seeds. On the twigs, there are adults and immature individuals, but also the queen and winged. The production of wings requires time and energy from the colony. The objective of this study was to investigate the presence of winged in ant colonies in twigs on the leaf litter. Our prediction is that the richness and abundance of winged in twigs are the greatest in rainy months. We collected all twigs with ants in 552 plots with 16 m2, totaling 8,832 m2 of leaf litter, in areas located in the Brazilian Atlantic Domain. -

ORIGINAL ARTICLE a Novel Intracellular Mutualistic Bacterium in the Invasive Ant Cardiocondyla Obscurior
The ISME Journal (2016) 10, 376–388 © 2016 International Society for Microbial Ecology All rights reserved 1751-7362/16 www.nature.com/ismej ORIGINAL ARTICLE A novel intracellular mutualistic bacterium in the invasive ant Cardiocondyla obscurior Antonia Klein1,8, Lukas Schrader1,8, Rosario Gil2, Alejandro Manzano-Marín2, Laura Flórez3, David Wheeler5, John H Werren6, Amparo Latorre2,7, Jürgen Heinze1, Martin Kaltenpoth3,4, Andrés Moya2 and Jan Oettler1 1Institut für Zoologie, Universität Regensburg, Regensburg, Germany; 2Institut Canvanilles de Biodiversitat i Biologia Evolutiva (ICBiBE), Parc Cientific de la Universitat de Valencia, Paterna (Valencia), Spain; 3Max Planck Institute for Chemical Ecology, Jena, Germany; 4Johannes Gutenberg University Mainz, Institute for Zoology, Department for Evolutionary Ecology, Mainz, Germany; 5Institute of Fundamental Sciences, Massey University, Palmerston North, New Zealand; 6Department of Biology, University Rochester, Rochester, NY, USA and 7Área de Genómica y Salud de la Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana (FISABIO)–Salud Pública, Valencia, Spain The evolution of eukaryotic organisms is often strongly influenced by microbial symbionts that confer novel traits to their hosts. Here we describe the intracellular Enterobacteriaceae symbiont of theinvasiveantCardiocondyla obscurior, ‘Candidatus Westeberhardia cardiocondylae’. Upon metamorphosis, Westeberhardia is found in gut-associated bacteriomes that deteriorate following eclosion. Only queens maintain Westeberhardia in the ovarian nurse cells from where the symbionts are transmitted to late-stage oocytes during nurse cell depletion. Functional analyses of the streamlined genome of Westeberhardia (533 kb, 23.41% GC content) indicate that neither vitamins nor essential amino acids are provided for the host. However, the genome encodes for an almost complete shikimate pathway leading to 4-hydroxyphenylpyruvate, which could be converted into tyrosine by the host. -

Field Guide of Common Ants
J U L Y / A U G 2 0 0 3 N P M A L I B R A R Y U P D A T E Insert this update into the NPMA Pest Management Library, which can be Updatepurchased from the Resource Center. phone: 703-573-8330 fax: 703-573-4116 Common Ants: A Pull-Out Guide for Use in the Field This Library Update is designed to of acrobat ants. Workers are 1/16” to 1/8” assist technicians in identification and in length and are normally brown to control of ants while servicing accounts. black in color. The pedicel, or front joint This update is not designed for of the abdomen has two nodes or instruction in basic ant biology, segments. Looking down on the ant nomenclature of the anatomy of the ant, under hand lens magnification, one pair or to be used as a key. For more detailed of spines is found on the thorax. Various information on those topics, refer to the species of acrobat ants are found Field Guide or other technical materials. throughout the United States. In the field, a great aid to identification Acrobat ants are named for their is the use of a hand lens. Many of these tendency to raise their abdomens in the ants are small and positive identification air when disturbed. As the abdomen is is not easy without a hand lens. This heart shaped and is frequently black and article focuses on common, non-wood- shiny, the acrobat motion is readily destroying ants, including: acrobat, observable. -

The Case of the Argentine Ant in Vineyards of Northern Argentina
insects Article Growing Industries, Growing Invasions? The Case of the Argentine Ant in Vineyards of Northern Argentina Maria Schulze-Sylvester 1,* ID , José A. Corronca 1 ID and Carolina I. Paris 2 1 FCN-IEBI (Instituto para el Estudio de la Biodiversidad de Invertebrados), CONICET CCT-Salta, Facultad de Ciencias Naturales, Universidad Nacional de Salta, Av. Bolivia 5150, CP 4400 Salta, Argentina; [email protected] 2 Departamento de Ecología, Genética y Evolución, Facultad de Ciencias Exactas y Naturales, Universidad Nacional de Buenos Aires, 1428 Buenos Aires, Argentina; [email protected] * Correspondence: [email protected]; Tel.: +54-387-425-5472 Received: 10 December 2017; Accepted: 23 January 2018; Published: 29 January 2018 Abstract: The invasive Argentine ant causes ecological and economic damage worldwide. In 2011, this species was reported in vineyards of Cafayate, a wine-producing town in the Andes, Argentina. While the local xeric climate is unsuitable for Argentine ants, populations could establish in association with vineyards where human activity and irrigation facilitate propagule introduction and survival. In 2013–2014, we combined extensive sampling of the area using ant-baits with monitoring of the change in land use and vineyard cultivated area over the past 15 years. Our results revealed that the species has thus far remained confined to a relatively isolated small area, owing to an effective barrier of dry shrublands surrounding the infested vineyards; yet the recent expansion of vineyard acreage in this region will soon connect this encapsulated area with the rest of the valley. When this happens, vulnerable ecosystems and the main local industry will be put at risk. -

US EPA-Pesticides; Disodium Octaborate Tetrahydrate
EFFICACY REVIEW DATE: IN 4-30-01 OUT 6-21-01 FILE OR REG. NO. 69529-1 PETITION OR EXP. PERMIT NO. DATE DIV. RECEIVED April 19, 2001 DATE OF SUBMISSION April 18, 2001 DATE SUBMISSION ACCEPTED TYPE PRODUCT(S): (I,)D, H, F, N, R, S DATA ACCESSION NO(S). 453568-01;D274488;S596027;Case#046539;AC:301 PRODUCT MGR. NO. 03-Layne/Quarles PRODUCT NAME(S) Pestbor COMPANY NAME Quality Borate Company SUBMISSION PURPOSE Provide performance data in reprint supporting claims for Argentine ant, pharaoh ant and Tap- inoma melanocephalum for formulated products. CHEMICAL & FORMULATION Disodium octaborate tetrahydrate 98% (30 lbs./cu.ft. bulk density manufacturing concentrate) CONCLUSIONS & RECOMMENDATIONS The data presented in EPA Accession (MRID) Number 453568-01, having been obtained from the reprint art- icle titled “Laboratory Evaluation of a Boric Acid Liquid Bait on Colonies of Tapinoma melanocephalum, Argentine Ants and Pharaoh Ants (Hymenoptera: Formicidae)S, which meets the requirements of § 11(b)(1)-(7) on p. 268 and the standard of § 95-11(c)(3)(b) on pp. 270-1 of the Product Performance Guidelines, are adequate to sup- port the registration of the subject product, the sole use for this formulation being for the manufacturing of end use insecticidal products, more specifically baits. The cited data (to be contin'd) is far more than is necessary to establish usefulness of this form- ulation for the making of insecticidal baits. Results with the 3 species included in the testing were as follows: Tapinoma melanocephalum workers were reduced by 97% -

Above-Belowground Effects of the Invasive Ant Lasius Neglectus in an Urban Holm Oak Forest
U B Universidad Autónoma de Barce lona Departamento de Biología Animal, de Biología Vegetal y de Ecología Unidad de Ecología Above-belowground effects of the invasive ant Lasius neglectus in an urban holm oak forest Tesis doctoral Carolina Ivon Paris Bellaterra, Junio 2007 U B Universidad Autónoma de Barcelona Departamento de Biología Animal, de Biología Vegetal y de Ecología Unidad de Ecología Above-belowground effects of the invasive ant Lasius neglectus in an urban holm oak forest Memoria presentada por: Carolina Ivon Paris Para optar al grado de Doctora en Ciencias Biológicas Con el Vº. Bº.: Dr Xavier Espadaler Carolina Ivon Paris Investigador de la Unidad de Ecología Doctoranda Director de tesis Bellaterra, Junio de 2007 A mis padres, Andrés y María Marta, y a mi gran amor Pablo. Agradecimientos. En este breve texto quiero homenajear a través de mi más sincero agradecimiento a quienes me ayudaron a mejorar como persona y como científica. Al Dr Xavier Espadaler por admitirme como doctoranda, por estar siempre dispuesto a darme consejos tanto a nivel profesional como personal, por darme la libertad necesaria para crecer como investigadora y orientarme en los momentos de inseguridad. Xavier: nuestras charlas más de una vez trascendieron el ámbito académico y fue un gustazo escucharte y compartir con vos algunos almuerzos. Te prometo que te enviaré hormigas de la Patagonia Argentina para tu deleite taxonómico. A Pablo. ¿Qué puedo decirte mi amor qué ya no te haya dicho? Gracias por la paciencia, el empuje y la ayuda que me diste en todo momento. Estuviste atento a los más mínimos detalles para facilitarme el trabajo de campo y de escritura. -

Population Dynamics of Invasive Argentine Ant
BioInvasions Records (2021) Volume 10, Issue 2: 467–476 CORRECTED PROOF Research Article Population dynamics of invasive Argentine ant Linepithema humile Mayr, 1868 (Hymenoptera: Formicidae) haplotypes in Kobe Port, Japan, and implications for the prediction of future dispersal and effective management Yugo Seko1,#,*, Yu Maebara2,#, Naoyuki Nakahama3,4, Takuya Nakamori1, Naoto Ishiwaka5, Yuki Morikawa5, Daisuke Hayasaka1,* and Takuo Sawahata1 1Graduate school of Agriculture, Kindai University, 3327-204, Nakamachi, Nara City, Nara Prefecture, Japan 2Nagoya Branch Office, Nippon Koei Co. Ltd., 1-20-22, Aoi, Naka-ku, Nagoya City, Aichi Prefecture, Japan 3Institute of Natural and Environmental Sciences, University of Hyogo, 6 Yayoigaoka, Sanda City, Hyogo Prefecture, Japan 4Division of Ecological Restoration, The Museum of Nature and Human Activities, 6 Yayoigaoka, Sanda City, Hyogo Prefecture, Japan 5Faculty of Agriculture, Kindai University, 3327-204, Nakamachi, Nara City, Nara Prefecture, Japan Author e-mails: [email protected] (YS), [email protected] (YMa), [email protected] (NN), [email protected] (TN), [email protected] (NI), [email protected] (YMo), [email protected]; [email protected] (DH), [email protected] (TS) #These authors contributed equally *Corresponding authors Citation: Seko Y, Maebara Y, Nakahama N, Nakamori T, Ishiwaka N, Morikawa Y, Abstract Hayasaka D, Sawahata T (2021) Population dynamics of invasive Argentine The Argentine ant Linepithema humile Mayr, native to South America, has been ant Linepithema humile Mayr, 1868 unintentionally introduced worldwide. Although L. humile usually forms an extremely (Hymenoptera: Formicidae) haplotypes in large supercolony all sharing the same haplotype among members in its introduced Kobe Port, Japan, and implications for the ranges, four haplotypes (LH1, LH2, LH3, LH4) with different genetic structures prediction of future dispersal and effective management. -

Argentine Ant
Pest Profile Photo credit: J.A. MacGown, Mississippi Entomological Museum, Mississippi State University Common Name: Argentine Ant Scientific Name: Linepithema humile Order and Family: Order Hymenoptera; Family Formicidae Size and Appearance: Length (mm) Appearance Egg Larva/Nymph Adult Workers: 2 -3 Shiny and smooth surface; light to dark brown with large eyes. mm They have a one-part waist with a well-developed node. These Queens: 4 – 6 ants lack a sting. mm Queens and males have wings. Queens resemble workers but are larger. Pupa (if applicable) Type of feeder (Chewing, sucking, etc.): Chewing Host(s): The Argentine ants feed primarily on sweet foods including plant nectar and honeydew from hemipterans such as aphids, which feed on plant juices. They also feed on other arthropods, especially ants and other insects, and small vertebrates. Description of Damage (larvae and adults): This species is an invasive and widespread pest with significant impacts on arthropod, vertebrate, and plant communities, therefore disrupting ecosystems. They also have significant impacts on agriculture and urban and residential areas. These ants often occur in high densities, especially in urban areas where water is available. These ants do come into homes in search of food and water. They tend and protect aphid and scale pests on a variety of plants from which the ants get honeydew. The Argentine ant is an invasive species, native of South America. This species has spread to the southern USA and is currently found in Arizona, California, Illinois, Maryland, Missouri, Oregon, Texas, and Washington states. References: Argentine ant, Texas Invasive Species. (2014). Retrieved from http://www.tsusinvasives.org/home/database/linepithema-humile Fisher, B.L. -

Genetic Mechanisms Underlying the Evolutionary Success of Eusocial Insects
insects Review (Epi)Genetic Mechanisms Underlying the Evolutionary Success of Eusocial Insects Kayli R. Sieber 1 , Taylor Dorman 1, Nicholas Newell 1 and Hua Yan 1,2,* 1 Department of Biology, University of Florida, Gainesville, FL 32611, USA; kayli.sieber@ufl.edu (K.R.S.); taylor.dorman@ufl.edu (T.D.); nicholas.newell@ufl.edu (N.N.) 2 Center for Smell and Taste, University of Florida, Gainesville, FL 32611, USA * Correspondence: hua.yan@ufl.edu; Tel.: +1-352-273-4983 Simple Summary: Social insects, namely ants, bees, and termites, are among the most numerous and successful animals on Earth. This is due to a variety of features: highly cooperative behavior performed by colony members and their specialization on a variety of tasks. Diverse physiological and behavioral specializations are regulated not only by the genetic system, but also by the epige- netic system which alters gene expressions without modifying the genetic code. This review will summarize recent advancements in such studies in eusocial insects. Abstract: Eusocial insects, such as bees, ants, and wasps of the Hymenoptera and termites of the Blattodea, are able to generate remarkable diversity in morphology and behavior despite being genetically uniform within a colony. Most eusocial insect species display caste structures in which reproductive ability is possessed by a single or a few queens while all other colony members act Citation: Sieber, K.R.; Dorman, T.; as workers. However, in some species, caste structure is somewhat plastic, and individuals may Newell, N.; Yan, H. (Epi)Genetic switch from one caste or behavioral phenotype to another in response to certain environmental cues. -
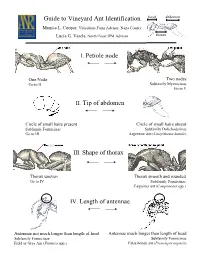
I. Petiole Node II. Tip of Abdomen IV. Length of Antennae Guide to Vineyard Ant Identification III. Shape of Thorax
Guide to Vineyard Ant Identification head abdomen Monica L. Cooper, Viticulture Farm Advisor, Napa County Lucia G. Varela, North Coast IPM Advisor thorax I. Petiole node One Node Two nodes Go to II Subfamily Myrmicinae Go to V II. Tip of abdomen Circle of small hairs present Circle of small hairs absent Subfamily Formicinae Subfamily Dolichoderinae Go to III Argentine Ant (Linepithema humile) III. Shape of thorax Thorax uneven Thorax smooth and rounded Go to IV Subfamily Formicinae Carpenter ant (Camponotus spp.) IV. Length of antennae Antennae not much longer than length of head Antennae much longer than length of head Subfamily Formicinae Subfamily Formicinae Field or Gray Ant (Formica spp.) False honey ant (Prenolepis imparis) head abdomen Petiole with two nodes Subfamily Myrmicinae (V-VIII) thorax V. Dorsal side of Thorax & Antennae One pair of spines on thorax No spines on thorax 12 segmented antennae 10 segmented antennae Go to VI Solenopsis molesta and Solenopsis xyloni VI. Underside of head No brush of bristles Brush of long bristles Go to VII Harvester ants (Pogonomyrmex californicus and P. brevispinosis) VII. Head and Thorax With hairs Without hairs Go to VIII Cardiocondyla mauritanica VIII. Head and Thorax With many parallel furrows Without parallel furrows Profile of thorax rounded Profile of thorax not evenly rounded Pavement ant (Tetramorium “species E”) Pheidole californica Argentine Ant (Linepithema humile), subfamily Dolichoderinae Exotic species 3-4 mm in length Deep brown to light black Move rapidly in distinct trails Feed on honeydew Shallow nests (2 inches from soil surface) Alex Wild Does not bite or sting Carpenter Ant (Camponotus spp.), subfamily Formicinae Large ant: >6 mm in length Dark color with smooth, rounded thorax Workers most active at dusk and night One of most abundant and widespread genera worldwide Generalist scavengers and predators: feed on dead and living insects, nectar, fruit juices and Jack K. -

Behavioral and Genetic Differentiation Between Native and Introduced Populations of the Argentine Ant
Biological Invasions 1: 43–53, 1999. © 1999 Kluwer Academic Publishers. Printed in the Netherlands. Behavioral and genetic differentiation between native and introduced populations of the Argentine ant ∗ Andrew V. Suarez , Neil D. Tsutsui, David A. Holway & Ted J. Case Department of Biology 0116, University of California at San Diego, La Jolla, CA 92093-0116, USA; ∗Author for correspondence (e-mail: [email protected]; fax: +1-619-534-7108) Received 9 June 1998; accepted in revised form 22 February 1999 Key words: Argentine ant, biological invasions, competition, invasive ants, Linepithema humile, microsatellites, nestmate recognition, population bottleneck, unicoloniality Abstract In this paper, we examine the hypothesis that reduced intraspecific aggression underlies the competitive prowess of Argentine ants in their introduced range. Specifically, we test three predictions of this hypothesis by comparing the genetic diversity, behavior, and ecology of Argentine ants in their native range to introduced populations. Differences between native and introduced populations of Argentine ants were consistent with our predictions. Introduced populations of the Argentine ant appear to have experienced a population bottleneck at the time of introduction, as evidenced by much reduced variation in polymorphic microsatellite DNA markers. Intraspecific aggression was rare in introduced populations but was common in native populations. Finally, in contrast to the Argentine ant’s ecological dominance throughout its introduced range, it did not appear dominant in the native ant assemblages studied in Argentina. Together these results identify a possible mechanism for the widespread success of the Argentine ant in its introduced range. Introduction Studies that compare the ecology of invasive species between their introduced and native ranges can be par- Despite the widespread problems associated with bio- ticularly insightful. -

Hymenoptera: Formicidae: Dolichoderinae) from Colombia
Revista Colombiana de Entomología 37 (1): 159-161 (2011) 159 Scientific note The first record of the genus Gracilidris (Hymenoptera: Formicidae: Dolichoderinae) from Colombia Primer registro del género Gracilidris (Hymenoptera: Formicidae: Dolichoderinae) para Colombia ROBERTO J. GUERRERO1 and CATALINA SANABRIA2 Abstract: The dolichoderine ant genus Gracilidris and its sole species, G. pombero, are recorded for the first time for Colombia from populations from the foothills of the Colombian Amazon basin. Comments and hypotheses about the biogeography of the genus are discussed. Key words: Ants. Biodiversity. Caquetá. Colombian Amazon. Grazing systems. Resumen: El género dolicoderino de hormigas Gracilidris y su única especie, G. pombero, son registrados por primera vez para Colombia, de poblaciones provenientes del piedemonte de la cuenca Amazónica colombiana. Algunos comen- tarios e hipótesis sobre la biogeografía del género son discutidos. Palabras clave: Hormigas. Biodiversidad. Caquetá. Amazonas colombiano. Pasturas ganaderas. Introduction distribution of the genus Gracilidris in South America. We also discuss each of the dolichoderine genera that occur in Currently, dolichoderine ants (Hymenoptera: Formicidae: Colombia. Dolichoderinae) include 28 extant genera (Bolton et al. 2006; Fisher 2009) distributed in four tribes according to Methods the latest higher level classification of the ant subfamily Dolichoderinae (Ward et al. 2010). Eleven of those extant We separated G. pombero specimens from all ants collected genera occur in the New World: Bothriomyrmex, Dolicho- in the project “Amaz_BD: Biodiversidad de los paisajes derus, Liometopum, Tapinoma, and Technomyrmex have a Amazónicos, determinantes socio-económicos y produc- global distribution, while Anillidris, Azteca, Dorymyrmex, ción de bienes y servicios”. This research was carried out in Forelius, Gracilidris, and Linepithema are endemic to the Caquetá department located in the western foothills of the New World.