Body Size, Not Phylogenetic Relationship Or Residency, Drives Interspecific Dominance in a Little Pocket Mouse Community
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
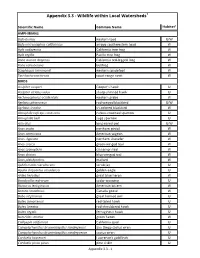
Appendix 3.3 - Wildlife Within Local Watersheds1
Appendix 3.3 - Wildlife within Local Watersheds1 2 Scientific Name Common Name Habitat AMPHIBIANS Bufo boreas western toad U/W Bufo microscaphus californicus arroyo southwestern toad W Hyla cadaverina California tree frog W Hyla regilla Pacific tree frog W Rana aurora draytonii California red-legged frog W Rana catesbeiana bullfrog W Scaphiopus hammondi western spadefoot W Taricha torosa torosa coast range newt W BIRDS Accipiter cooperi Cooper's hawk U Accipiter striatus velox sharp-shinned hawk U Aechmorphorus occidentalis western grebe W Agelaius phoeniceus red-winged blackbird U/W Agelaius tricolor tri-colored blackbird W Aimophila ruficeps canescens rufous-crowned sparrow U Aimophilia belli sage sparrow U Aiso otus long-eared owl U/W Anas acuta northern pintail W Anas americana American wigeon W Anas clypeata northern shoveler W Anas crecca green-winged teal W Anas cyanoptera cinnamon teal W Anas discors blue-winged teal W Anas platrhynchos mallard W Aphelocoma coerulescens scrub jay U Aquila chrysaetos canadensis golden eagle U Ardea herodius great blue heron W Bombycilla cedrorum cedar waxwing U Botaurus lentiginosus American bittern W Branta canadensis Canada goose W Bubo virginianus great horned owl U Buteo jamaicensis red-tailed hawk U Buteo lineatus red-shouldered hawk U Buteo regalis ferruginous hawk U Butorides striatus green heron W Callipepla californica California quail U Campylorhynchus brunneicapillus sandiegensis San Diego cactus wren U Campylorhynchus brunneicapillus sandiegoense cactus wren U Carduelis lawrencei Lawrence's -

Jeremy Langley Chief Operations Officer Wilhite Langley, Inc. 21800 Barton Road, Ste 102 Grand Terrace, Ca 92313
47 1st Street, Suite 1 Redlands, CA 92373-4601 (909) 915-5900 May 22, 2019 Jeremy Langley Chief Operations Officer Wilhite Langley, Inc. 21800 Barton Road, Ste 102 Grand Terrace, Ca 92313 RE: Biological Resources Assessment, Jurisdictional Waters Delineation Glen Helen/Devore parcel - APN: 0261-161-17, Devore, CA Dear Mr. Langley: Jericho Systems, Inc. (Jericho) is pleased to provide this letter report that details the results of a general Biological Resources Assessment (BRA) that includes habitat suitability assessments for nesting birds, Burrowing owl (Athene cunicularia) [BUOW] and a Jurisdictional Waters Delineation (JD) for the proposed Glen Helen/Devore parcel (Project) located within Assessor’s Parcel Number (APN) #0261-161-17 in the community of Devore, CA (Attachment B: Figures 1 and 2). This report is designed to address potential effects of the proposed Project to designated Critical Habitats and/or any species currently listed or formally proposed for listing as endangered or threatened under the federal Endangered Species Act (ESA) and the California Endangered Species Act (CESA), or species designated as sensitive by the California Department of Fish and Wildlife (CDFW), or the California Native Plant Society (CNPS). Attention was focused on sensitive species known to occur locally. This report also addresses resources protected under the Migratory Bird Treaty Act, federal Clean Water Act (CWA) regulated by the U.S. Army Corps of Engineers (USACE) and Regional Water Quality Control Board (RWQCB) respectively; and Section 1602 of the California Fish and Game Code (FCG) administered by the CDFW. SITE LOCATION The approximately 1-acre parcel (APN: 0261-161-17) is located north of Kendall Drive just north of the intersection with N. -

Mammal Species Native to the USA and Canada for Which the MIL Has an Image (296) 31 July 2021
Mammal species native to the USA and Canada for which the MIL has an image (296) 31 July 2021 ARTIODACTYLA (includes CETACEA) (38) ANTILOCAPRIDAE - pronghorns Antilocapra americana - Pronghorn BALAENIDAE - bowheads and right whales 1. Balaena mysticetus – Bowhead Whale BALAENOPTERIDAE -rorqual whales 1. Balaenoptera acutorostrata – Common Minke Whale 2. Balaenoptera borealis - Sei Whale 3. Balaenoptera brydei - Bryde’s Whale 4. Balaenoptera musculus - Blue Whale 5. Balaenoptera physalus - Fin Whale 6. Eschrichtius robustus - Gray Whale 7. Megaptera novaeangliae - Humpback Whale BOVIDAE - cattle, sheep, goats, and antelopes 1. Bos bison - American Bison 2. Oreamnos americanus - Mountain Goat 3. Ovibos moschatus - Muskox 4. Ovis canadensis - Bighorn Sheep 5. Ovis dalli - Thinhorn Sheep CERVIDAE - deer 1. Alces alces - Moose 2. Cervus canadensis - Wapiti (Elk) 3. Odocoileus hemionus - Mule Deer 4. Odocoileus virginianus - White-tailed Deer 5. Rangifer tarandus -Caribou DELPHINIDAE - ocean dolphins 1. Delphinus delphis - Common Dolphin 2. Globicephala macrorhynchus - Short-finned Pilot Whale 3. Grampus griseus - Risso's Dolphin 4. Lagenorhynchus albirostris - White-beaked Dolphin 5. Lissodelphis borealis - Northern Right-whale Dolphin 6. Orcinus orca - Killer Whale 7. Peponocephala electra - Melon-headed Whale 8. Pseudorca crassidens - False Killer Whale 9. Sagmatias obliquidens - Pacific White-sided Dolphin 10. Stenella coeruleoalba - Striped Dolphin 11. Stenella frontalis – Atlantic Spotted Dolphin 12. Steno bredanensis - Rough-toothed Dolphin 13. Tursiops truncatus - Common Bottlenose Dolphin MONODONTIDAE - narwhals, belugas 1. Delphinapterus leucas - Beluga 2. Monodon monoceros - Narwhal PHOCOENIDAE - porpoises 1. Phocoena phocoena - Harbor Porpoise 2. Phocoenoides dalli - Dall’s Porpoise PHYSETERIDAE - sperm whales Physeter macrocephalus – Sperm Whale TAYASSUIDAE - peccaries Dicotyles tajacu - Collared Peccary CARNIVORA (48) CANIDAE - dogs 1. Canis latrans - Coyote 2. -

Draft Environmental Assessment of Marine Geophysical Surveys by the R/V Marcus G. Langseth for the Southern California Collaborative Offshore Geophysical Survey
DRAFT ENVIRONMENTAL ASSESSMENT OF MARINE GEOPHYSICAL SURVEYS BY THE R/V MARCUS G. LANGSETH FOR THE SOUTHERN CALIFORNIA COLLABORATIVE OFFSHORE GEOPHYSICAL SURVEY Submitted to: National Science Foundation Division of Ocean Sciences 4201 Wilson Blvd., Suite 725 Arlington, VA 22230 Submitted by: Scripps Institution of Oceanography, UCSD 8675 Discovery Way La Jolla, CA 92023 Contact: Professor Neal Driscoll 858.822.5026; [email protected] Prepared by: Padre Associates, Inc. 5290 Overpass Road, Suite 217 Goleta, CA 93113 June 2012 Southern California Collaborative Offshore Geophysical Survey (SCCOGS) Environmental Assessment TABLE OF CONTENTS 1.0 PURPOSE AND NEED ................................................................................................... 1 2.0 ALTERNATIVES INCLUDING PROPOSED ACTION ...................................................... 6 2.1 PROPOSED ACTION ......................................................................................... 6 2.2 PROJECT LOCATION ........................................................................................ 6 2.3 PROJECT ACTIVITIES ....................................................................................... 6 2.3.1 Mobilization and Demobilization .............................................................. 9 2.3.2 Offshore Survey Operations .................................................................... 9 2.3.2.1 Survey Vessel Specifications ..................................................... 10 2.3.2.2 Air Gun Description ................................................................... -

LOS ANGELES POCKET MOUSE Perognathus Longimembris Brevinasus
Terrestrial Mammal Species of Special Concern in California, Bolster, B.C., Ed., 1998 111 Los Angeles pocket mouse, Perognathus longimembris brevinasus Philip V. Brylski Description: This is a small heteromyid rodent, averaging about 113 mm TL with weight from 8 to 11 g. The Los Angeles pocket mouse can be potentially confused only with juveniles of the sympatric California pocket mouse (Chaetodipus californicus), from which it can be distinguished by the absence of spiny hairs in the dorsal pelage and the absence of a distinct crest on the tail. Pelage is buff above and white below. Many of the dorsal hairs are black-tipped, giving the pelage a "salt and pepper" appearance, similar to but lighter than that of the Pacific pocket mouse (P. l. pacificus). Like all silky pocket mice, there is usually a small white spot at the anterior base of the ear, and an indistinct larger buff spot behind the ear, the plantar surface of the hindfeet are naked or lightly haired, and the lateral hairs of the hind toes project anteriorly and laterally, resulting in a "fringed-toed" effect, which may enhance locomotor efficiency on sandy substrates. Taxonomic Remarks: This is one of eight subspecies of the little pocket mouse (P. longimembris) in California (Hall 1981). P. l. brevinasus was first described by Osgood (1900) as a race of the Panamint pocket mouse (P. panamintinus). Both brevinasus and panamintinus were arranged as subspecies of P. longimembris by Huey (1928). An important taxonomic character of brevinasus is its short rostrum, a character also shared by pacificus. P. -

San Bernardino Kangaroo Rat Trapping Study
NATURAL RESOURCES ASSESSMENT, INC. San Bernardino Kangaroo Rat (Dipodomys merriami parvus) Presence/Absence Trapping Studies Cajon Boulevard Warehouse Project San Bernardino, California Prepared for: L&L Environmental 721 Nevada, Suite 307 Redlands, CA 92373 951 681 4929 Prepared by: ENVIRA P. O. Box 2612 Ramona, CA 92065 Phone 619-885-0236 E-mail [email protected] May 9, 2018 Project Number: LLE18-101 T (951) 686-4483 3415 Valencia Hill Drive F (951) 686-8418 Riverside, California 92507 [email protected] Cajon Boulevard Warehouse Development! NATURAL RESOURCES ASSESSMENT, INC. San Bernardino Kangaroo Rat Trapping Survey CERTIFICATION This Phase One Survey and report were conducted and prepared in accordance with professional requirements for small mammal trapping studies by Philippe Vergne (USFWS Permit TE068072-3). Philippe Jean Vergne, Field Biologist and Author. !!!!!!! !! May 9, 2018 Cajon Warehouse Trapping LLE18-101!i Cajon Boulevard Warehouse Development! NATURAL RESOURCES ASSESSMENT, INC. San Bernardino Kangaroo Rat Trapping Survey Table of Contents Page 1.0 Introduction!.........................................................................................................................................................................1 2.0 Site Location and Project Description!..............................................................................................................................1 3.0 Methods................................................................................................................................................................................! -

2021 Rangewide SKR Management & Monitoring Plan
Stephens’ Kangaroo Rat Rangewide Management and Monitoring Plan Photo by Moose Peterson March 2021 Prepared by Conservation Biology Institute for Bureau of Land Management and Riverside County Habitat Conservation Agency CBI is a 501(c)3 tax-exempt organization that works collaboratively to conserve biological diversity in its natural state through applied research, education, planning, and community service. Preferred Citation: Spencer, W.D., D. DiPietro, H. Romsos, D. Shier, and R. Chock. 2021. Stephens’ Kangaroo Rat Rangewide Management and Monitoring Plan. Unpublished report prepared by the Conservation Biology Institute for Bureau of Land Management and Riverside County Habitat Conservation Agency. March 2021. SKR Rangewide Management & Monitoring Plan Conservation Biology Institute, 2021 Table of Contents Foreword 5 Acknowledgments 7 Glossary 8 1. Introduction 12 1.1. Background and Context 14 1.2. Approach 16 1.2.1. Use of Habitat Models and Delineating Population Units 17 1.2.2. Biogeographic Mapping and Genetic Considerations 17 1.2.3. Threats Assessment 18 1.2.4. Management Strategy 18 1.2.5. Monitoring Strategy 18 1.2.6. Data Management Strategy 19 1.2.7. Coordination Structure 19 2. SKR Ecology 20 2.1. Distribution and Population Genetics 20 2.2. Habitat 21 2.3. Sociality and Burrow Use 22 2.4. Diet and Foraging 23 2.5. Space-use Patterns 23 2.6. Reproduction 23 2.7. Communication 24 2.8. Activity Patterns 24 2.9. Interspecific Relationships 25 3. SKR Habitat Model 27 3.1. Methods 28 3.2. Results and Discussion 29 4. Delineating SKR Habitat & Population Units 34 4.1. -

Recovery Research for the Endangered Pacific Pocket Mouse: an Overview of Collaborative Studies1
Recovery Research for the Endangered Pacific Pocket Mouse: An Overview of Collaborative Studies1 Wayne D. Spencer2 Abstract The critically endangered Pacific pocket mouse (Perognathus longimembris pacificus), feared extinct for over 20 years, was “rediscovered” in 1993 and is now documented at four sites in Orange and San Diego Counties, California. Only one of these sites is considered large enough to be potentially self-sustaining without active intervention. In 1998, I gathered a team of biologists to initiate several research tasks in support of recovery planning for the species. The PPM Studies Team quickly determined that species recovery would require active trans- locations or reintroductions to establish new populations, but that we knew too little about the biology of P. l. pacificus and the availability of translocation receiver sites to design such a program. Recovery research from 1998 to 2000 therefore focused on (1) a systematic search for potential translocation receiver sites; (2) laboratory and field studies on non-listed, surro- gate subspecies (P. l. longimembris and P. l. bangsi) to gain biological insights and perfect study methods; (3) studies on the historic and extant genetic diversity of P. l. pacificus; and (4) experimental habitat manipulations to increase P. l. pacificus populations. Using existing geographic information system (GIS) data, we identified sites throughout the historic range that might have appropriate soils and vegetation to support translocated P. l. pacificus. Re- connaissance surveys of habitat value were completed in all large areas of potential habitat identified by the model. Those sites having the highest habitat potential are being studied with more detailed and quantitative field analyses. -

Nocturnal Rodents
Nocturnal Rodents Peter Holm Objectives (Chaetodipus spp. and Perognathus spp.) and The monitoring protocol handbook (Petryszyn kangaroo rats (Dipodomys spp.) belong to the 1995) states: “to document general trends in family Heteromyidae (heteromyids), while the nocturnal rodent population size on an annual white-throated woodrats (Neotoma albigula), basis across a representative sample of habitat Arizona cotton rat (Sigmodon arizonae), cactus types present in the monument”. mouse (Peromyscus eremicus), and grasshopper mouse (Onychomys torridus), belong to the family Introduction Muridae. Sigmodon arizonae, a native riparian Nocturnal rodents constitute the prey base for species relatively new to OPCNM, has been many snakes, owls, and carnivorous mammals. recorded at the Dos Lomitas and Salsola EMP All nocturnal rodents, except for the grasshopper sites, adjacent to Mexican agricultural fields. mouse, are primary consumers. Whereas Botta’s pocket gopher (Thomomys bottae) is the heteromyids constitute an important guild lone representative of the family Geomyidae. See of granivores, murids feed primarily on fruit Petryszyn and Russ (1996), Hoffmeister (1986), and foliage. Rodents are also responsible for Petterson (1999), Rosen (2000), and references considerable excavation and mixing of soil layers therein, for a thorough review. (bioturbation), “predation” on plants and seeds, as well as the dispersal and caching of plant seeds. As part of the Sensitive Ecosystems Project, Petryszyn and Russ (1996) conducted a baseline Rodents are common in all monument habitats, study originally titled, Special Status Mammals are easily captured and identified, have small of Organ Pipe Cactus National Monument. They home ranges, have high fecundity, and respond surveyed for nocturnal rodents and other quickly to changes in primary productivity and mammals in various habitats throughout the disturbance (Petryszyn 1995, Petryszyn and Russ monument and found that murids dominated 1996, Petterson 1999). -

Mammalian Species Surveys in the Acquisition Areas on the Tejon Ranch, California
MAMMALIAN SPECIES SURVEYS IN THE ACQUISITION AREAS ON THE TEJON RANCH, CALIFORNIA PREPARED FOR THE TEJON RANCH CONSERVANCY Prepared by: Brian L. Cypher, Christine L. Van Horn Job, Erin N. Tennant, and Scott E. Phillips California State University, Stanislaus Endangered Species Recovery Program One University Circle Turlock, CA 95382 August 16, 2010 esrp_2010_TejonRanchsurvey.doc MAMMALIAN SPECIES SURVEYS IN THE ACQUISITION AREAS ON THE TEJON RANCH, CALIFORNIA TABLE OF CONTENTS Introduction ......................................................................................................................... 1 Study Areas ......................................................................................................................... 3 Methods............................................................................................................................... 4 Target Special Status Species .................................................................................................................... 4 Camera Station Surveys ............................................................................................................................. 4 Live-Trapping ............................................................................................................................................ 5 Spotlight Surveys ....................................................................................................................................... 5 Opportunistic Observations ...................................................................................................................... -

Stephens' Kangaroo Rat Survey
Stephens’ Kangaroo Rat Survey and Management Recommendations for the Santa Ysabel Open Space Reserve San Diego County, California Prepared by Wayne D. Spencer, Ph.D. 815 Madison Avenue San Diego, California 92116 Prepared for The Nature Conservancy 3033 Fifth Avenue, Suite 105 San Diego, California 92103 August 2002 Santa Ysabel Open Space Reserve SKR Conservation Biology Institute (CBI) is a 501(c)3 tax-exempt research and 1/3/2005 planning institution. We work collabo ratively to help conserve biological 2 diversity through research, educatio n, planning, and community service. Santa Ysabel Open Space Reserve SKR Introduction The endangered Stephens’ kangaroo rat (SKR; Dipodomys stephensi) is restricted largely to the San Jacinto Valley and vicinity in western Riverside County, but San Diego County supports a few scattered populations. The sparse grasslands this species requires are already rare in San Diego County, and remaining grasslands are increasingly threatened by development. Most potential habitat on private lands in San Diego County has never been surveyed for SKR, so additional populations may exist. Even currently unoccupied habitat may be essential to maintaining and recovering populations of this rare species by providing dispersal corridors and areas for population expansion. Grasslands in and near the Santa Ysabel Valley, in central San Diego County, appear to have strong potential to support SKR, and may play an important role in long-term species viability by maintaining habitat connectivity between known SKR populations. The Nature Conservancy (TNC) recently purchased for open space preservation two properties that include grassland habitat along the edges of the Santa Ysabel Valley. These properties, formerly comprising the Edwards Ranch, are to be managed by the County of San Diego as the Santa Ysabel Open Space Reserve (SYOSR). -

Southern California Weekend, 2017
Southern California Weekend Venkat Sankar March 31-April 3, 2017 This weekend trip across the coast, mountains, and deserts of S California was designed to take advantage of abnormally high winter rains following on years of “drought” in this part of the state. The trip ran better than expected, netting 26 species and good sightings of almost all target species (my only major dips were Cactus Mouse, Canyon Mouse, and amazingly Round-tailed Ground Squirrel—what I thought was my easiest target!). The only significant surprise was the lower numbers of kangaroo rats and pocket mice at most sites than expected (given the wet winter); perhaps it will be better to search for these in early-mid May. Thank you to Vladimir Dinets for advice on species IDs and Brian Keelan for details on the Stonehouse Mine. (Day 1: 3/31/17) We departed the Bay Area about an hour late and hit commute traffic driving towards the I-5, leaving us too far behind schedule to attempt a detour to Munz Ranch Road near Lake Hughes. After an early dinner, we made our first stop to break the boredom on San Emigdio Canyon Road at about 8:30 PM, which we drove to the gate at the Wind Wolves Preserve entrance. In about 30 minutes, we observed 4 Heermann’s Kangaroo Rats (in the grasslands), 2 almost certain Agile Kangaroo Rats (in a brushy wash with darker tail stripes and larger ears than the former; I have prior experience with this species), and best of all – 3 San Joaquin Pocket Mice (now the best site I know for this species).