The Journal of Wildlife Management, 83(7)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
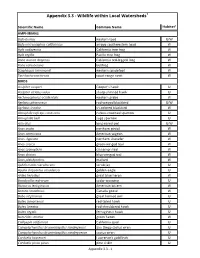
Appendix 3.3 - Wildlife Within Local Watersheds1
Appendix 3.3 - Wildlife within Local Watersheds1 2 Scientific Name Common Name Habitat AMPHIBIANS Bufo boreas western toad U/W Bufo microscaphus californicus arroyo southwestern toad W Hyla cadaverina California tree frog W Hyla regilla Pacific tree frog W Rana aurora draytonii California red-legged frog W Rana catesbeiana bullfrog W Scaphiopus hammondi western spadefoot W Taricha torosa torosa coast range newt W BIRDS Accipiter cooperi Cooper's hawk U Accipiter striatus velox sharp-shinned hawk U Aechmorphorus occidentalis western grebe W Agelaius phoeniceus red-winged blackbird U/W Agelaius tricolor tri-colored blackbird W Aimophila ruficeps canescens rufous-crowned sparrow U Aimophilia belli sage sparrow U Aiso otus long-eared owl U/W Anas acuta northern pintail W Anas americana American wigeon W Anas clypeata northern shoveler W Anas crecca green-winged teal W Anas cyanoptera cinnamon teal W Anas discors blue-winged teal W Anas platrhynchos mallard W Aphelocoma coerulescens scrub jay U Aquila chrysaetos canadensis golden eagle U Ardea herodius great blue heron W Bombycilla cedrorum cedar waxwing U Botaurus lentiginosus American bittern W Branta canadensis Canada goose W Bubo virginianus great horned owl U Buteo jamaicensis red-tailed hawk U Buteo lineatus red-shouldered hawk U Buteo regalis ferruginous hawk U Butorides striatus green heron W Callipepla californica California quail U Campylorhynchus brunneicapillus sandiegensis San Diego cactus wren U Campylorhynchus brunneicapillus sandiegoense cactus wren U Carduelis lawrencei Lawrence's -

Body Size, Not Phylogenetic Relationship Or Residency, Drives Interspecific Dominance in a Little Pocket Mouse Community
Animal Behaviour 137 (2018) 197e204 Contents lists available at ScienceDirect Animal Behaviour journal homepage: www.elsevier.com/locate/anbehav Body size, not phylogenetic relationship or residency, drives interspecific dominance in a little pocket mouse community * Rachel Y. Chock a, , Debra M. Shier a, b, Gregory F. Grether a a Department of Ecology & Evolutionary Biology, University of California, Los Angeles, CA, U.S.A. b Recovery Ecology, San Diego Zoo Institute for Conservation Research, Escondido, CA, U.S.A. article info The role of interspecific aggression in structuring ecological communities can be important to consider Article history: when reintroducing endangered species to areas of their historic range that are occupied by competitors. Received 6 September 2017 We sought to determine which species is the most serious interference competitor of the endangered Initial acceptance 6 November 2017 Pacific pocket mouse, Perognathus longimembris pacificus, and more generally, whether interspecific Final acceptance 1 December 2017 aggression in rodents is predicted by body size, residency status or phylogenetic relatedness. We carried out simulated territory intrusion experiments between P. longimembris and four sympatric species of MS. number: A17-00719 rodents (Chaetodipus fallax, Dipodomys simulans, Peromyscus maniculatus, Reithrodontomys megalotis)ina field enclosure in southern California sage scrub habitat. We found that body size asymmetries strongly Keywords: predicted dominance, regardless of phylogenetic relatedness or the residency status of the individuals. aggression The largest species, D. simulans, was the most dominant while the smallest species, R. megalotis, was the dominance least dominant to P. longimembris. Furthermore, P. longimembris actively avoided encounters with all interference competition Perognathus longimembris species, except R. -

Paleontological Resources of the Upper and Middle San Pedro Valley
Paleontological Resources of the Upper and Middle San Pedro Valley Robert D. McCord Arizona Museum of Natural History Geological setting Regional extension causing block faulting – creation of the Basin and Range ~15Ma Poorly developed drainage results in lakes in valley bottom ?-3.4 Ma Drainage develops with flow to north, marshes, ponds and lakes significant from time to time Early Pleistocene Saint David Formation ? – 3.4 million lakes, few fossils Well developed paleomagnetic timeframe – a first for terrestrial sediments! Succession of faunas from ~3 to 1.5 Ma Blancan to ? Irvingtonian NALMA Plants diatoms charophytes Equisetum (scouring rush) Ostracoda (aquatic crustaceans) Cypridopsis cf. vidua Limnocythere cf. staplini Limnocythere sp. Candona cf. renoensis Candona sp. A Candona sp. B ?Candonlella sp. ?Heterocypris sp. ?Cycloypris sp. Potamocypris sp. Cyprideis sp. Darwinula sp. Snails and a Clam Pisidium casertanum (clam) Fossaria dalli (aquatic snail) Lymnaea caperata (aquatic snail) Lymnaea cf. elodes (aquatic snail) Bakerilymnaea bulimoides (aquatic snail) Gyraulus parvus (aquatic snail) Promenetus exacuous (aquatic snail) Promenetus umbilicatellus (aquatic snail) Physa virgata (aquatic snail) Gastrocopta cristata (terrestrial snail) Gastrocopta tappaniana (terrestrial snail) Pupoides albilabris (terrestrial snail) Vertigo milium (terrestrial snail) Vertigo ovata (terrestrial snail) cf. Succinea (terrestrial snail) Deroceras aenigma (slug) Hawaila minuscula (terrestrial snail) Fish and Amphibians indeterminate small fish Ambystoma tigrinum (tiger salamander) Scaphiopus hammondi (spadefoot toad) Bufo alvarius (toad) Hyla eximia (tree frog) Rana sp. (leopard frog) Turtles and Lizards Kinosternon arizonense (mud turtle) Terrapene cf. ornata (box turtle) Gopherus sp. (tortoise) Hesperotestudo sp. (giant tortoise) Eumeces sp. (skink) “Cnemidophorus” sp. (whiptail lizard) Crotaphytus sp. (collared lizard) Phrynosoma sp. (horned lizard) Sceloporus sp. -

Draft Environmental Assessment of Marine Geophysical Surveys by the R/V Marcus G. Langseth for the Southern California Collaborative Offshore Geophysical Survey
DRAFT ENVIRONMENTAL ASSESSMENT OF MARINE GEOPHYSICAL SURVEYS BY THE R/V MARCUS G. LANGSETH FOR THE SOUTHERN CALIFORNIA COLLABORATIVE OFFSHORE GEOPHYSICAL SURVEY Submitted to: National Science Foundation Division of Ocean Sciences 4201 Wilson Blvd., Suite 725 Arlington, VA 22230 Submitted by: Scripps Institution of Oceanography, UCSD 8675 Discovery Way La Jolla, CA 92023 Contact: Professor Neal Driscoll 858.822.5026; [email protected] Prepared by: Padre Associates, Inc. 5290 Overpass Road, Suite 217 Goleta, CA 93113 June 2012 Southern California Collaborative Offshore Geophysical Survey (SCCOGS) Environmental Assessment TABLE OF CONTENTS 1.0 PURPOSE AND NEED ................................................................................................... 1 2.0 ALTERNATIVES INCLUDING PROPOSED ACTION ...................................................... 6 2.1 PROPOSED ACTION ......................................................................................... 6 2.2 PROJECT LOCATION ........................................................................................ 6 2.3 PROJECT ACTIVITIES ....................................................................................... 6 2.3.1 Mobilization and Demobilization .............................................................. 9 2.3.2 Offshore Survey Operations .................................................................... 9 2.3.2.1 Survey Vessel Specifications ..................................................... 10 2.3.2.2 Air Gun Description ................................................................... -

LOS ANGELES POCKET MOUSE Perognathus Longimembris Brevinasus
Terrestrial Mammal Species of Special Concern in California, Bolster, B.C., Ed., 1998 111 Los Angeles pocket mouse, Perognathus longimembris brevinasus Philip V. Brylski Description: This is a small heteromyid rodent, averaging about 113 mm TL with weight from 8 to 11 g. The Los Angeles pocket mouse can be potentially confused only with juveniles of the sympatric California pocket mouse (Chaetodipus californicus), from which it can be distinguished by the absence of spiny hairs in the dorsal pelage and the absence of a distinct crest on the tail. Pelage is buff above and white below. Many of the dorsal hairs are black-tipped, giving the pelage a "salt and pepper" appearance, similar to but lighter than that of the Pacific pocket mouse (P. l. pacificus). Like all silky pocket mice, there is usually a small white spot at the anterior base of the ear, and an indistinct larger buff spot behind the ear, the plantar surface of the hindfeet are naked or lightly haired, and the lateral hairs of the hind toes project anteriorly and laterally, resulting in a "fringed-toed" effect, which may enhance locomotor efficiency on sandy substrates. Taxonomic Remarks: This is one of eight subspecies of the little pocket mouse (P. longimembris) in California (Hall 1981). P. l. brevinasus was first described by Osgood (1900) as a race of the Panamint pocket mouse (P. panamintinus). Both brevinasus and panamintinus were arranged as subspecies of P. longimembris by Huey (1928). An important taxonomic character of brevinasus is its short rostrum, a character also shared by pacificus. P. -

Breeding Biology and Diet of the Ferruginous Hawk in South Dakota
Wilson Bull., 94(l), 1982, pp. 4654 BREEDING BIOLOGY AND DIET OF THE FERRUGINOUS HAWK IN SOUTH DAKOTA CHARLES L. BLAIR AND FRANK SCHITOSKEY, JR. The Ferruginous Hawk (Buteo regalis) is the largest North American buteo. It occurs throughout most of the western United States and breeds from Alberta to western Texas and from Washington to Arizona. In this study we investigated the breeding biology of the Ferruginous Hawks in northwest South Dakota, complimenting earlier studies by Weston (1969) in Utah, Olendorff (1973) in Colorado, Howard (1975) in Utah and Idaho, and Lokemoen and Duebbert (1976) in north-central South Dakota. STUDY AREA AND METHODS The study area encompassed about 7000 km ’ in northwest South Dakota, including all of Harding County. The area is semi-arid and has a mid-continental climate with long, cold winters and short, warm summers (Spuhler et al. 1971). Eighty-five percent of Harding County is rangeland dominated by western wheatgrass (Agropyron smithii) and needle grass (Stipa comata). Sagebrush (Artemesia spp.) occurs throughout the area and is widespread in the western third of the county. Small grain crops compose 9% of the area, pastureland and tame hay 3% and woodland 3%. Elevated table lands are dominated by ponderosa pine (Pinw ponderma) Savannah, whereas green ash (Fraxinus pennsylvanicas), willow (S&x spp.) and Siberian elm (Ulmas pumila) predominate in riparian areas and ravines. Two or more biologists conducted a daily census of birds on the study area in 1976 and 1977; Ferruginous Hawk studies began the day the first hawk was sighted. Active nests were located either during aerial surveys at altitudes of 150-175 m, or by ground searches from roads. -

Recovery Research for the Endangered Pacific Pocket Mouse: an Overview of Collaborative Studies1
Recovery Research for the Endangered Pacific Pocket Mouse: An Overview of Collaborative Studies1 Wayne D. Spencer2 Abstract The critically endangered Pacific pocket mouse (Perognathus longimembris pacificus), feared extinct for over 20 years, was “rediscovered” in 1993 and is now documented at four sites in Orange and San Diego Counties, California. Only one of these sites is considered large enough to be potentially self-sustaining without active intervention. In 1998, I gathered a team of biologists to initiate several research tasks in support of recovery planning for the species. The PPM Studies Team quickly determined that species recovery would require active trans- locations or reintroductions to establish new populations, but that we knew too little about the biology of P. l. pacificus and the availability of translocation receiver sites to design such a program. Recovery research from 1998 to 2000 therefore focused on (1) a systematic search for potential translocation receiver sites; (2) laboratory and field studies on non-listed, surro- gate subspecies (P. l. longimembris and P. l. bangsi) to gain biological insights and perfect study methods; (3) studies on the historic and extant genetic diversity of P. l. pacificus; and (4) experimental habitat manipulations to increase P. l. pacificus populations. Using existing geographic information system (GIS) data, we identified sites throughout the historic range that might have appropriate soils and vegetation to support translocated P. l. pacificus. Re- connaissance surveys of habitat value were completed in all large areas of potential habitat identified by the model. Those sites having the highest habitat potential are being studied with more detailed and quantitative field analyses. -

Nocturnal Rodents
Nocturnal Rodents Peter Holm Objectives (Chaetodipus spp. and Perognathus spp.) and The monitoring protocol handbook (Petryszyn kangaroo rats (Dipodomys spp.) belong to the 1995) states: “to document general trends in family Heteromyidae (heteromyids), while the nocturnal rodent population size on an annual white-throated woodrats (Neotoma albigula), basis across a representative sample of habitat Arizona cotton rat (Sigmodon arizonae), cactus types present in the monument”. mouse (Peromyscus eremicus), and grasshopper mouse (Onychomys torridus), belong to the family Introduction Muridae. Sigmodon arizonae, a native riparian Nocturnal rodents constitute the prey base for species relatively new to OPCNM, has been many snakes, owls, and carnivorous mammals. recorded at the Dos Lomitas and Salsola EMP All nocturnal rodents, except for the grasshopper sites, adjacent to Mexican agricultural fields. mouse, are primary consumers. Whereas Botta’s pocket gopher (Thomomys bottae) is the heteromyids constitute an important guild lone representative of the family Geomyidae. See of granivores, murids feed primarily on fruit Petryszyn and Russ (1996), Hoffmeister (1986), and foliage. Rodents are also responsible for Petterson (1999), Rosen (2000), and references considerable excavation and mixing of soil layers therein, for a thorough review. (bioturbation), “predation” on plants and seeds, as well as the dispersal and caching of plant seeds. As part of the Sensitive Ecosystems Project, Petryszyn and Russ (1996) conducted a baseline Rodents are common in all monument habitats, study originally titled, Special Status Mammals are easily captured and identified, have small of Organ Pipe Cactus National Monument. They home ranges, have high fecundity, and respond surveyed for nocturnal rodents and other quickly to changes in primary productivity and mammals in various habitats throughout the disturbance (Petryszyn 1995, Petryszyn and Russ monument and found that murids dominated 1996, Petterson 1999). -

Mammalian Species Surveys in the Acquisition Areas on the Tejon Ranch, California
MAMMALIAN SPECIES SURVEYS IN THE ACQUISITION AREAS ON THE TEJON RANCH, CALIFORNIA PREPARED FOR THE TEJON RANCH CONSERVANCY Prepared by: Brian L. Cypher, Christine L. Van Horn Job, Erin N. Tennant, and Scott E. Phillips California State University, Stanislaus Endangered Species Recovery Program One University Circle Turlock, CA 95382 August 16, 2010 esrp_2010_TejonRanchsurvey.doc MAMMALIAN SPECIES SURVEYS IN THE ACQUISITION AREAS ON THE TEJON RANCH, CALIFORNIA TABLE OF CONTENTS Introduction ......................................................................................................................... 1 Study Areas ......................................................................................................................... 3 Methods............................................................................................................................... 4 Target Special Status Species .................................................................................................................... 4 Camera Station Surveys ............................................................................................................................. 4 Live-Trapping ............................................................................................................................................ 5 Spotlight Surveys ....................................................................................................................................... 5 Opportunistic Observations ...................................................................................................................... -

Kangaroo Rat and Pocket Mouse
Shrew Family Order Rodentia (Soricoidae) masked shrew vagrant shrew water shrew Sorex cinereus Sorex vagrans Sorex palustris grassland streambank streambank Mouse, Vole, Rats, and Muskrat (Cricetidae) meadow vole long-tailed vole heather vole Microtus pennsylvanicu Microtus longicaudus Phenacomys intermedius grassland streambank streambank/grassland/mountain Gapper’s red-backed vole deer mouse Western harvest mouse Clethrionomys gapperi Peromycus maniculatus Reithrodontomys megalotis mountain mountain/streambank grassland bushy-tailed woodrat Neotoma cinerea mountain rock mouse Northern grasshopper mouse Peromyscus difficilis Onychomys leucogaster mountain grassland Jumping Mouse Kangaroo Rat and Family Pocket Mouse silky pocket mouse (Zapodidae) (Heteromyidae) Perognathus flavus desert Western jumping mouse Ord’s kangaroo rat Apache pocket mouse Zapus princeps Dipodomys ordii Perognathus apache streambank desert mountain 1:1 0 1 2 3 4 5 6 inches 1 - Rodents Tracks are actual size. Pocket Gopher Porcupine Family Order Rodentia Family (Erethizonidae) (Geomyidae) Beaver Family (Castoridae) porcupine Erethizon dorsatum mountains/grasslands scale 1:3 beaver Castor canadensis streams/lakes/wetlands Northern pocket gopher scale 1:3 Thomomys talpoides grasslands scale 1:1 1:3 0 1 2 3 4 5 6 inches Squirrel Family (Sciuridae) least chipmunk Colorado chipmunk chicaree Eutamias minimus Eutamias quadrivittatus Tamiasciurus douglassi mountain/grassland mountain forest Abert’s squirrel Sciurus aberti kaibabensis mountain/forest rock ground squirrel golden-mantled ground Spermophilus variegatus squirrel mountain Spermophilus lateralis streambank yellow-bellied marmot Gunnison’s prairie dog thirteen-lined ground squirrel Marmota flaviventris Cynomys gunnisoni Spermophilus tridecemlineatus mountain/rockslide grassland grassland 1:1 0 1 2 3 4 5 6 inches 2 - Rodents Rodentia tracks vary in size. Sciuridae tracks are actual size. -

Chaetodipus Baileyi) and the Peromyscus Eremicus Species Group: Historical Vicariance of the Baja California Peninsular Desert Brett R
Molecular Phylogenetics and Evolution Vol. 17, No. 2, November, pp. 161–172, 2000 doi:10.1006/mpev.2000.0842, available online at http://www.idealibrary.com on Comparative Phylogeography of Baileys’ Pocket Mouse (Chaetodipus baileyi) and the Peromyscus eremicus Species Group: Historical Vicariance of the Baja California Peninsular Desert Brett R. Riddle,* David J. Hafner,† and Lois F. Alexander* *Department of Biological Sciences, University of Nevada at Las Vegas, 4505 Maryland Parkway, Las Vegas, Nevada 89154-4004; and †New Mexico Museum of Natural History, 1801 Mountain Road NW, Albuquerque, New Mexico 87104 Received October 13, 1999; revised July 24, 2000 Nelson and E. A. Goldman surveyed the Peninsula on Phylogenetic analysis of 699 bp of the mitochondrial horseback for the U. S. Bureau of Biological Survey in DNA (mtDNA) COIII and 450 bp of the cytochrome b 1905 and 1906 (Nelson, 1921). Huey (1964) provided a genes among 14 species of coarse-haired pocket mice description of the Peninsula’s mammals as of 1960, and (Heteromyidae: Chaetodipus) corroborated previous a general description of the mammalian biogeography indications that genetic divergence between species of the region by Orr (1960) was followed by more de- and species groups within the genus is generally very tailed analyses of the ecological (Lawlor, 1983a,b) and high, suggesting old times of divergence, and that the historical biogeography of the mammals of the Penin- nominal species C. baileyi represents a highly diver- sular mainland and surrounding islands (Hafner and gent lineage within the genus, with no closely related Riddle, 1997). In concert with these studies have been extant sister species. -

2Nd Owl Symposium Importance of Prairie
2nd Owl Symposium Importance of Prairie Wetlands and Avian Prey to Breeding Great Horned Owls (Bubo virginianus) in Northwestern North Dakota Robert K. Murphy 1 Abstract.—Prey use by Great Horned Owls (Bubo virginianus) is documented widely in North America, but not in the vast northern Great Plains. During spring through early summer 1986-1987, I recorded 2,900 prey items at 22 Great Horned Owl nesting areas in the prairie pothole farm- and rangelands of northwestern North Dakota. The owls relied heavily on wetland-dependent prey species (overall, 57 percent by number and 76 percent biomass) especially ducks (Anserinae) and rails (Rallidae). Far more avian (65 percent by number and 84 percent biomass) and less mammalian prey were used than typically reported. Variation in diet composition among owl families was not explained well by nesting area habitat, and was dominated by prey from wetlands regardless of wetland habitat availability. Diets of Great Horned Owls (Bubo virginianus) (Murphy 1993, Sargeant et al. 1993). The are better documented than those of most increase in this generalist predator may have other North American strigiforms. The owl implications for population dynamics of species preys mainly on small to mid-size mammals on which it preys. My objectives were to quan- especially small rodents and leporids tify diet composition of breeding Great Horned (Errington et al. 1940; Korschgen and Stuart Owls in an area of mixed farm- and rangeland 1972; McInvaille and Keith 1974; Marti and in the northern Great Plains, to assess varia- Kochert 1995, 1996; Voous 1988) although its tion in prey use among owl pairs, and to test list of prey includes diverse sizes and taxa (see whether such variation is explained by habitat Bent 1938).