Gradient Growth in Western Pond Turtles (Actinemys Marmorata) Across Thermal Gradients
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effectiveness of Nest Site Restoration for the Endangered Northern Map Turtle
MD-16- SHA-TU-1-03 Larry Hogan, Governor Pete K. Rahn, Secretary Boyd K. Rutherford, Lt. Governor Gregory C. Johnson, P. E., Administrator Maryland Department of Transportation STATE HIGHWAY ADMINISTRATION RESEARCH REPORT Effectiveness of Nest Site Restoration for the Endangered Northern Map Turtle Report 2: Use of Artificial Nesting Sites and Wildlife Exclusion Fence to Enhance Nesting Success Research Team Richard A. Seigel, Kaite Anderson, Brian Durkin, Jenna Cole, Sarah Cooke, and Teal Richards-Dimitrie TOWSON UNIVERSITY FINAL REPORT October 2016 The contents of this report reflect the views of the author who is responsible for the facts and the accuracy of the data presented herein. The contents do not necessarily reflect the official views or policies of the Maryland Department of Transportation’s State Highway Administration. This report does not constitute a standard, specification, or regulation. Technical Report Documentation Page 1. Report No. 2. Government Accession No. 3. Recipient's Catalog No. MD-15- SHA-TU-1-03 4. Title and Subtitle 5. Report Date Effectiveness of Nest Site Restoration for the Endangered Northern Map October, 2016 Turtle 6. Performing Organization Code Report 2: Use of Artificial Nesting Sites and Wildlife Exclusion Fence to Enhance Nesting Success 7. Author/s 8. Performing Organization Report No. Richard A Seigel, Kaite Anderson, Brian Durkin, Jenna Cole, Sarah Cooke, and Teal Richards-Dimitrie 9. Performing Organization Name and Address 10. Work Unit No. (TRAIS) Towson University 8000 York Road 11. Contract or Grant No. Towson, MD 21252 SP509B4M 12. Sponsoring Organization Name and Address 13. Type of Report and Period Covered Maryland State Highway Administration Final Report Office of Policy & Research 14. -

Graptemys Geographica), and Sympatric Invasive Red-Eared Slider Turtles (Trachemys Scripta Elegans), in the Upper Niagara River Brian E
State University of New York College at Buffalo - Buffalo State College Digital Commons at Buffalo State Biology Theses Biology 8-2015 Home Range, Habitat Use, and Movements of Native Northern Map Turtles (Graptemys geographica), and Sympatric Invasive Red-Eared Slider Turtles (Trachemys scripta elegans), in the Upper Niagara River Brian E. Haas State University of New York College at Buffalo, [email protected] Advisor Edward Standora, PhD First Reader Harold W. Avery, PhD Second Reader Christopher Pennuto, PhD Third Reader Robert Warren, PhD Department Chair I. Martha Skerrett, PhD To learn more about the Biology Department and its educational programs, research, and resources, go to http://biology.buffalostate.edu/. Recommended Citation Haas, Brian E., "Home Range, Habitat Use, and Movements of Native Northern Map Turtles (Graptemys geographica), and Sympatric Invasive Red-Eared Slider Turtles (Trachemys scripta elegans), in the Upper Niagara River" (2015). Biology Theses. 16. http://digitalcommons.buffalostate.edu/biology_theses/16 Follow this and additional works at: http://digitalcommons.buffalostate.edu/biology_theses Part of the Behavior and Ethology Commons, and the Biology Commons Home range, habitat use, and movements of native northern map turtles (Graptemys geographica), and sympatric invasive red-eared slider turtles (Trachemys scripta elegans), in the Upper Niagara River by Brian Haas An Abstract of a Thesis in Biology Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Arts August 2015 Buffalo State College State University of New York Department of Biology 2 ABSTRACT OF THESIS Home range, habitat use, and movements of native northern map turtles (Graptemys geographica), and sympatric invasive red-eared slider turtles (Trachemys scripta elegans), in the Upper Niagara River Turtle populations throughout the world are in decline due to the effects associated with anthropogenic disturbances. -

Turtles of the Upper Mississippi River System
TURTLES OF THE UPPER MISSISSIPPI RIVER SYSTEM Tom R. Johnson and Jeffrey T. Briggler Herpetologists Missouri Department of Conservation Jefferson City, MO March 27, 2012 Background: A total of 13 species and subspecies of turtles are known to live in the Upper Mississippi River, its backwaters and tributaries. There are a few species that could be found occasionally, but would likely account for less than 5% of the species composition of any area. These species are predominantly marsh animals and are discussed in a separate section of this paper. For additional information on turtle identification and natural history see Briggler and Johnson (2006), Christiansen and Bailey (1988), Conant and Collins (1998), Ernst and Lovich (2009), Johnson (2000), and Vogt (1981). This information is provided to the fisheries field staff of the LTRM project so they will be able to identify the turtles captured during fish monitoring. The most current taxonomic information of turtles was used to compile this material. The taxonomy followed in this publication is the Scientific and Standard English Names of Amphibians and Reptiles of North America North of Mexico, with comments regarding confidence in our understanding (6th edition) by Crothers (2008). Species Identification, Natural History and Distribution: What follows is a synopsis of the 13 turtle species and subspecies which are known to occur in the Upper Mississippi River environs. Species composition changes between the upper and lower reaches of the LTRM study area (Wisconsin/Minnesota state line and southeastern Missouri) due to changes in aquatic habitats. For example, the Northern Map Turtle (Graptemys geographica) is abundant in the northern portion of the river with clearer water and abundant snail prey. -
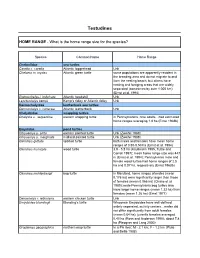
Home Range Testudines
Testudines HOME RANGE - What is the home range size for the species? Species Common Name Home Range Cheloniidae sea turtles Caretta c. caretta Atlantic loggerhead Unk Chelonia m. mydas Atlantic green turtle some populations are apparently resident in the breeding area and do not migrate to and from the nesting beach, but others have nesting and foraging areas that are widely separated (sometimes by over 1,000 km) (Ernst et al. 1994) Eretmochelys i. imbricata Atlantic hawksbill Unk Lepidochelys kempii Kemp’s ridley or Atlantic ridley Unk Dermochelyidae leatherback sea turtles Dermochelys c. coriacea Atlantic leatherback Unk Chelydridae snapping turtles Chelydra s. serpentina eastern snapping turtle in Pennsylvanina, nine adults…had estimated home ranges averaging 1.8 ha (Ernst 1968b) Emydidae pond turtles Chrysemys p. picta eastern painted turtle Unk (Zweifel 1989) Chrysemys p. marginata midland painted turtle Unk (Zweifel 1989) Clemmys guttata spotted turtle both males and females have mean home ranges of 0.50-0.53 ha (Ernst et al. 1994) Clemmys insculpta wood turtle 3.9 - 5.8 ha (Kaufmann 1995, Tuttle and Carroll 1997); mean home range size was 447 m (Ernst et al. 1994); Pennsylvania male and female wood turtles had home ranges of 2.5 ha and 0.07 ha, respectively (Ernst 1968b) Clemmys muhlenbergii bog turtle in Maryland, home ranges of males (mean 0.176 ha) were significantly larger than those of females (mean 0.066 ha) (Chase et al. 1989); male Pennsylvania bog turtles also have larger home ranges (mean 1.33 ha) than females (mean 1.26 ha) (Ernst 1977) Deirochelys r. -

Herpetological Monitoring and Assessment on the Trinity River, Trinity County, California—Final Report
Prepared in cooperation with the Trinity River Restoration Program Herpetological Monitoring and Assessment on the Trinity River, Trinity County, California—Final Report Open-File Report 2016–1089 U.S. Department of the Interior U.S. Geological Survey Cover: Photographs of adult Foothill yellow-legged frog (Rana boylii) (top left); basking adult western pond turtle (Actinemys marmorata) (top right); juvenile western pond turtle (bottom left); and Foothill yellow-legged frog egg mass with embryo mortality (bottom right). Photographs by Don Ashton, U.S. Geological Survey, June 2014. Herpetological Monitoring and Assessment on the Trinity River, Trinity County, California—Final Report By Melissa L. Snover and Michael J. Adams Prepared in cooperation with the Trinity River Restoration Program Open-File Report 2016–1089 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior SALLY JEWELL, Secretary U.S. Geological Survey Suzette M. Kimball, Director U.S. Geological Survey, Reston, Virginia: 2016 For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment—visit http://www.usgs.gov/ or call 1–888–ASK–USGS (1–888–275–8747). For an overview of USGS information products, including maps, imagery, and publications, visit http://www.usgs.gov/pubprod/. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this information product, for the most part, is in the public domain, it also may contain copyrighted materials as noted in the text. Permission to reproduce copyrighted items must be secured from the copyright owner. -

Chelonian Advisory Group Regional Collection Plan 4Th Edition December 2015
Association of Zoos and Aquariums (AZA) Chelonian Advisory Group Regional Collection Plan 4th Edition December 2015 Editor Chelonian TAG Steering Committee 1 TABLE OF CONTENTS Introduction Mission ...................................................................................................................................... 3 Steering Committee Structure ........................................................................................................... 3 Officers, Steering Committee Members, and Advisors ..................................................................... 4 Taxonomic Scope ............................................................................................................................. 6 Space Analysis Space .......................................................................................................................................... 6 Survey ........................................................................................................................................ 6 Current and Potential Holding Table Results ............................................................................. 8 Species Selection Process Process ..................................................................................................................................... 11 Decision Tree ........................................................................................................................... 13 Decision Tree Results ............................................................................................................. -

(Graptemys Geographica) in Canada Northern Map Turtle
PROPOSED Species at Risk Act Management Plan Series Management Plan for the Northern Map Turtle (Graptemys geographica) in Canada Northern Map Turtle 2016 Recommended citation: Environment Canada. 2016. Management Plan for the Northern Map Turtle (Graptemys geographica) in Canada [Proposed]. Species at Risk Act Management Plan Series. Environment Canada, Ottawa. iv + 45 pp. For copies of the management plan, or for additional information on species at risk, including the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) Status Reports, residence descriptions, action plans, and other related recovery documents, please visit the Species at Risk (SAR) Public Registry1. Cover illustration: © Parks Canada Agency Également disponible en français sous le titre « Plan de gestion de la tortue géographique (Graptemys geographica) au Canada [Proposition] » © Her Majesty the Queen in Right of Canada, represented by the Minister of the Environment, 2016. All rights reserved. ISBN Catalogue no. Content (excluding the illustrations) may be used without permission, with appropriate credit to the source. 1 http://sararegistry.gc.ca/default.asp?lang=En&n=24F7211B-1 Management Plan for the Northern Map Turtle 2016 Preface The federal, provincial, and territorial government signatories under the Accord for the Protection of Species at Risk (1996)2 agreed to establish complementary legislation and programs that provide for effective protection of species at risk throughout Canada. Under the Species at Risk Act (S.C. 2002, c.29) (SARA), the federal competent ministers are responsible for the preparation of management plans for listed species of special concern and are required to report on progress within five years after the publication of the final document on the SAR Public Registry. -

Field Guide to the Turtles of Lake George
A Field Guide to the Turtles of the Lake George Watershed GEOR KE G A E L T T U C R E T J L O E R M P O N G IT O R I N A Field Guide to the Turtles of the Lake George Watershed was produced by the Lake George Assocation (LGA) in cooperation with the New York State Department of Environmental Conservation (NYS DEC) with generous funding support from the Helen V. Froelich Foundation. NYS DEC The Lake George Turtle Monitoring Project is a volunteer monitoring program. Trained volunteers monitor for six different species of turtles found in the Lake George watershed to determine presence, density, range, and habitat use of the turtles. The ultimate goal of the project is to increase current scientific GEOR KE G A E knowledge of the resident turtle species in the Lake George watershed, so that effective L T T U conservation measures can be implemented to ensure continued biodiversity. C R E T J L O E R M P ©2007 by the Lake George Association O N G IT O R I N Quick Reference to the Turtles of the Lake George Watershed John White John White John White Common Snapping Common Musk Spotted p.8-9 p.10-11 p.12-13 Karen Robbins John White John White GEOR KE G A E L T T U Wood C Eastern Painted Northern Map R E T J L O p.14-15 p.16-17 p.18-19 E 1 R M P O N G IT O R I N GEOR KE G A E L T T U C R E T J L O E 2 R M P O N G Lake George IT O R I N • Lake George is located in northern New York, in the southeastern part of the Adirondack Park. -

Tsp2011richards-Dimitrie.Pdf (2.367Mb)
TOWSON UNIVERSITY COLLEGE OF GRADUATE STUDIES AND RESEARCH Spatial ecology and diet of Maryland endangered Northern Map Turtles (Graptemys geographica) in an altered river system: Implications for conservation and management by Teal M. Richards-Dimitrie A thesis Presented to the faculty of Towson University in partial fulfillment of the requirements for the degree Master of Science in Biology April, 2011 Department of Biological Sciences Towson University Towson, Maryland 21252 Towson University College of Graduate Studies and Research TOWSON UNIVERSITY COLLEGE OF GRADUATE STUDIES AND RESEARCH Thesis Approval Page This is to certify that the thesis prepared by Teal M. Richards-Dimitrie entitled Spatial ecology and diet of Maryland endangered Northern Map Turtles (Graptemys geographica) in an altered river system: Implications for conservation and management has been approved by the thesis committee as satisfactorily completing the thesis requirements for the degree of Masters of Biology. ____________________________________ _________________ Chair, Thesis Committee Date ____________________________________ _________________ Committee Member Date ____________________________________ _________________ Committee Member Date ____________________________________ _________________ Dean, College of Graduate Studies and Research Date ii Acknowledgements There are many people I wish to thank for their support and contributions to my graduate experience. First and foremost I want to thank my advisor Richard Seigel. From our first meeting Rich has challenged me to be the critical thinking, professional biologist I wish to be. He helped me find a project with direct conservation implications which is something that was important to my wanting to do graduate level research. I have had many fantastic experiences beyond my thesis work and Rich encouraged all of them-truly caring about the success of my future. -

Mass Mortality of Northern Map Turtles ( Graptemys Geographica )
80 THE CANADIAN FIELD -N ATURALIST Vol. 129 Mass Mortality of Northern Map Turtles ( Graptemys geographica ) JORDAN CATRYSSE , E MILY SLAVIK 1, J ONATHAN CHOQUETTE 2, A SHLEY E. L EIFSO 2, and CHRISTINA M. D AVY 2, 3, 4 1Ontario Parks, 18050 Rondeau Park Road, RR 1 Morpeth, Ontario N0P 1X0 Canada 2Wildlife Preservation Canada, RR 5, 5420 Highway 6 North, Guelph, Ontario N1H 6J2 Canada 3Natural Resources DNA Profiling and Forensic Centre, Trent University, DNA Building, 2140 East Bank Drive, Peterborough, Ontario K9J 7B8 Canada 4Corresponding author: [email protected] Catrysse, Jordan, Emily Slavik, Jonathan Choquette, Ashley E. Leifso, and Christina M. Davy. 2015. Mass mortality of Northern Map Turtles ( Graptemys geographica ). Canadian Field-Naturalist 129(1): 80–83. We report a mass mortality of Northern Map Turtles ( Graptemys geographica [LeSueur, 1817]) on the north shore of Lake Erie, Ontario, Canada. Thirty-five dead adult females were recovered from a nesting area over a period of four weeks. Preda - tion and boat strikes were both excluded as potential cause of death, but the actual cause could not be determined because of the poor condition of the carcasses. Other possible explanations for the mortality include poisoning, drowning, and infection with an unidentified pathogen. Mass mortality in long-lived species, such as turtles, can have long-term effects on population growth and is a cause for concern in a species at risk. Key Words: Northern Map Turtle; Graptemys geographica ; mass mortality; Lake Erie; Ontario; Canada Introduction On 16 July 2012, J. C. walked along the beach at the In summer 2012, we documented a mass mortality nesting site and discovered two desiccated Northern of mature, female Northern Map Turtles ( Graptemys Map Turtle carcasses in sandy dunes well above the geographica [LeSueur, 1817]) at a marsh–dune com - waterline. -
2011 Petition to Protect Freshhwater Turtles Under CITES
Sent Via Email and Certified Mail Return Receipt Requested August 15, 2011 Division of Scientific Authority U.S. Fish and Wildlife Service 4401 North Fairfax Drive, Room 110 Arlington, VA 22203 [email protected] Phone: 703-358-1708 Fax: 703-358-2276 Re: Species Proposals for Consideration at CoP16 On behalf of the Center for Biological Diversity, I am writing to provide information and recommendations on freshwater turtle species that should be considered as candidates for U.S. proposals to amend Appendix II of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (“CITES”) at the upcoming sixteenth meeting of the Conference of the Parties (“CoP16”). See 76 Fed. Reg. 34746 (June 14, 2011) (requesting information and recommendations). The Center for Biological Diversity (“Center”) is a non-profit, public interest, conservation organization whose mission is to conserve imperiled native species and their habitat and to fulfill the continuing educational goals of its membership and the general public in the process. The United States is a key player in the international turtle trade with exports reaching millions of live turtles each year. Given the enormity of this commerce, the United States has a duty to take a leading role in promoting responsible commercial turtle trade. We urge the U.S. Fish and Wildlife Service (“FWS”) to propose at CoP16 that the following species be included in Appendix II: Alligator snapping turtle (Change Listing from Appendix III to Appendix II) Map turtles (Change Listing From Appendix III to Appendix II) Soft-shell turtles Spotted turtle Blanding’s turtle Diamondback terrapin In addition, we request that FWS list the common snapping turtle on Appendix III, although inclusion of a species in Appendix III is a unilateral decision that does not require a proposal to be brought forward to the Conference of Parties. -
The Tortuga Gazette and Education Since 1964 Volume 55, Number 1 • January/February 2019
Dedicated to CALIFORNIA TURTLE & TORTOISE CLUB Turtle & Tortoise Conservation, Preservation the Tortuga Gazette and Education Since 1964 Volume 55, Number 1 • January/February 2019 Pearl River map turtle, Graptemys pearlensis. Photo by Cris Hagen, United States Geological Survey. Public domain. Map turtle species, Graptemys spp. Agassiz, 1857 Map Turtles — an Overview by M. A. Cohen ndemic to North America, the family of basking, semiaquatic emydid turtles, map turtles are Ethirteen map turtle species chelonians, the common name of comparatively small. As a species, inhabit freshwater ecosystems Graptemys—map turtle—denotes map turtles display significant sex- throughout the eastern half of the the map-like markings, often ual dimorphism (differences related United States as well as southeast- resembling waterways on old maps, to the sex of the individual) with ern Canada. The genus Graptemys that occur on the species’ carapace. females being much larger than was officially described by the Graptemys species are also com- males. Female map turtles, at 7 to Swiss-American biologist/geolo- monly known as sawback turtles, 10 inches (18 to 25 centimeters) gist Jean Louis Rodolphe Agassiz in referring to the distinctive keel, in carapace length, are often up to 1857. running the medial length of the twice the length of males and can The genus name Graptemys derives carapace, which is typically ser- possess up to ten times the body from two Greek root words: grapt-, rated and spiked. Map turtles are mass of males. At 3 to 7 inches which means inscribed or written, also known for the elaborate strip- (8 to 18 centimeters) in carapace and -emys, meaning a turtle or ing on their heads, limbs, and tails.