Food Hygiene in Aseptic Processing and Packaging Systems
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

How to Make a Smell Training Kit July 3 2019
Some frequently asked questions Q. How much oil do I need in the jar? A. You only need enough to saturate the paper disc. Any more than that is just a waste of the oil. Q. I can’t smell anything! Have I done it wrong? A. Probably not. If you’ve followed the directions, your jars should be plenty “smelly”. The saturated disc, kept in the closed space with the cap on the jar, creates a really strong smell. If you are not smelling it now, give it time. Q. Can I put my nose all the way into the jar? A. That is not recommended. Keep the tip of your nose out of the jar. Q. What if I want to reuse the jar, but with different oils? A. You can do this, but you need to give the jars a really Smell Training Kits good clean with hot water and soap. Let them dry thoroughly. The lid will smell like the previous oil (not great, but you could improvise and remove the inside of the cap, which is made of white, plastic coated paper). Then cut yourself some new watercolour paper discs and make up the new jars. How to make your own Q. Can I use cotton pads inside the jars? A. Cotton pads are not recommended. They make a great place for bacteria to collect. Watercolour paper is absorbent, but does not harbour bacteria. Contact details E: [email protected] • W: abscent.org © AbScent is a charity registered in England and Wales No. 1183468• • Registered Office: 14 London Street, Andover, Hampshire SP10 2PA © AbScent 2019 Making your own kit is easy Just follow these simple steps. -

Food Packaging (FS 522 / FS 495) Aseptic Processing and Packaging
Food Packaging (FS 522 / FS 495) Aseptic Processing and Packaging Components of an aseptic processing system Aseptic processing is a thermal process in which the product and container are sterilized separately and brought together in a sterile environment. It involves pumping, deaeration, and sterilization of a food product, followed by holding it for a specified period of time (in a holding tube -- required to have a 1/4" rise per foot length of tube), cooling it, and finally packaging it in a sterile container. The use of high temperature for a short period of time (in comparison with conventional canning) in aseptic processing yields a high quality product. Care should be taken to ensure that all process calculations are performed after the deaeration stage and not based on the initial raw product. Deaeration is accomplished in a vessel maintained at a certain degree of vacuum by means of a vacuum pump. The product is fed into the vessel at 55 - 70 /C through a nozzle at the center of the vessel. Vacuum is controlled to obtain a product flash of about 5 /C. An internal spiral condenser condenses vapors and other condensable gases. The deaerated product is discharged through the bottom and pumped to the heating section. Another important part of an aseptic processing system is the back pressure valve which provides sufficient pressure to prevent boiling of the product at processing temperatures which can be as high as 125-130 /C. An aseptic surge tank provides the means for product to be continuously processed even if the packaging system is not operational due to any malfunction. -

WHO Good Manufacturing Practices for Sterile Pharmaceutical Products
© World Health Organization WHO Technical Report Series, No. 961, 2011 Annex 6 WHO good manufacturing practices for sterile pharmaceutical products Introduction Following implementation of these WHO good manufacturing practices (GMP) guidelines (1) within the context of the WHO Prequalifi cation of Medicines Programme, clarifying, editorial modifi cations have been proposed. These changes were adopted for maintenance purposes. In order to ease reading the full guideline has been reproduced again as an Annex to the current report of the WHO Expert Committee on Specifi cations for Pharmaceutical Preparations. WHO good manufacturing practices for sterile pharmaceutical products 1. General considerations 2. Quality control 3. Sanitation 4. Manufacture of sterile preparations 5. Sterilization 6. Ter minal sterilization 7. Aseptic processing and sterilization by fi ltration 8. Isolator technology 9. Blow/fi ll/seal technology 10. Personnel 11. Premises 12. Equipment 13. Finishing of sterile products References Further reading 261 1. General considerations 1.1 The production of sterile preparations should be carried out in clean areas, entry to which should be through airlocks for personnel and/or for equipment and materials. Clean areas should be maintained to an appropriate standard of cleanliness and supplied with air that has passed through fi lters of the required effi ciency. 1.2 The various operations of component preparation (such as those involving containers and closures), product preparation, fi lling and sterilization should be carried out in separate areas within the clean area. These areas are classifi ed into four grades (see section 4). 1.3 Manufacturing operations are divided here into two categories: —fi rst, those where the product is terminally sterilized; and — second, those which are conducted aseptically at some or all stages. -

Guide for Labeling Consumer Package by Weight, Volume, Count, Or Measure (Length, Area Or Thickness)
NIST Special Publication 1020 Guide for Labeling Consumer Package by Weight, Volume, Count, or Measure (length, area or thickness) Editors: David Sefcik Lisa Warfield This publication is available free of charge from: https://doi.org/10.6028/NIST.SP.1020 NIST Special Publication 1020 Guide for Labeling Consumer Package by Weight, Volume, Count, or Measure (length, area or thickness) Editors: David Sefcik Lisa Warfield Dr. Douglas Olson, Chief Office of Weights and Measures Physical Measurement Laboratory This publication is available free of charge from: https://doi.org/10.6028/NIST.SP.1020 June 2020 NIST SP 1020 supersedes all previous editions U.S. Department of Commerce Wilbur L. Ross, Jr., Secretary National Institute of Standards and Technology Walter Copan, NIST Director and Undersecretary of Commerce for Standards and Technology Certain commercial entities, equipment, or materials may be identified in this document in order to describe an experimental procedure or concept adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the entities, materials, or equipment are necessarily the best available for the purpose. National Institute of Standards and Technology Special Publication 1020 Natl. Inst. Stand. Technol. Spec. Publ. 1020, 40 pages (June 2020) This publication is available free of charge from: https://doi.org/10.6028/NIST.SP.1020 Foreword This document, “Guide for Labeling Consumer Packages by Weight, Volume, Count, or Measure (length, area, or thickness),” is based on the Uniform Packaging and Labeling Regulation (UPLR) in National Institute of Standards and Technology Handbook 130, “Uniform Laws and Regulation in the Areas of Legal Metrology and Fuel Quality.” It provides a summary of labeling requirements for consumer products and commodities sold by weight, volume, count, or measure. -

Gas Generator Bottle Introduction SCIENTIFIC This Gas Generator Setup Provides an Easy Way to Generate and Collect Gas
Gas Generator Bottle Introduction SCIENTIFIC This gas generator setup provides an easy way to generate and collect gas. Specific instructions are provided for the generation of hydrogen gas using zinc and acid. Concepts • Generation of gases • Water displacement Materials Hydrochloric acid solution, HCl, 3 M Glass plates or Sulfuric acid solution, H2SO4, 3 M Glass tubing Mossy zinc, Zn, 6 g Pneumatic trough Water, tap Rubber tubing Bent glass tubing* Silicone grease packet* Gas collecting bottles or tubes, 3 or 4 Thistle tube* Gas generator bottle* Two-hole rubber stopper* *Materials included. Safety Precautions Hydrochloric acid solution is toxic by ingestion and inhalation and is severely corrosive to skin, eyes and other tissues, as is sulfuric acid solu- tion. Hydrogen gas is a highly flammable gas and a severe fire hazard. Exercise extreme caution when testing the gas and keep the gas generator away from flames. Wear chemical splash goggles, chemical-resistant gloves, and a chemical-resistant apron. This activity requires the use of hazardous components and/or has the potential for hazardous reactions. Please review current Material Safety Data Sheets for additional safety, handling, and disposal information. Procedure 1. Set up the apparatus as shown in the figure to the right. Lubricate the glass tubing and thistle tube with silicone grease before inserting into the stopper. Make sure Thistle tube the water level is above the platform. Prepare bottles for collecting gas by water Two-hole rubber stopper displacement. To do this, fill each gas collecting bottle (or tube) over the brim with tap water, and then cover each with a flat glass plate. -

Trace Elements Specimen Collection Guide
500 Chipeta Way Salt Lake City, Utah 84108-1221 Phone: 801-583-2787 | toll free: 800-242-2787 A nonprofit enterprise of the University of Utah and its Department of Pathology Fax: 801-522-2706 | aruplab.com TRACE ELEMENTS SPECIMEN COLLECTION GUIDE Analysis for trace elements at ARUP is performed in a clean laboratory environment that includes a system of positive- pressure and HEPA-filtered air. Since detection limits for many trace elements are calculated in parts per billion, this helps minimize environmental contamination of specimens. Additionally, contamination control during specimen collection provides more accurate and clinically useful results. Note: Refer to the ARUP Laboratory Test Directory for specific instructions when collecting specimens for cadmium exposure testing (http://ltd.aruplab.com/Tests/Pub/0025013). Urine Collection Tissue Although it is nearly impossible to obtain a completely Follow information on individual pages of the laboratory uncontaminated urine specimen, steps can be taken to test directory. minimize environmental contamination. Avoid collecting the specimen in an area where Whole Blood, Serum, and Red Blood Cell Collection Materials comprising the tube and the tube top are a environmental contamination is likely to occur. In an consideration. Rubber stoppers in collection tubes other industrial or construction setting, it is important that than the royal blue tube are known to contain elements clothing worn in the workplace be removed prior to that can contaminate a specimen. Contamination can be specimen collection to prevent dust on the clothing introduced as the needle punctures the stopper in a tube from contaminating the specimen. other than royal blue. Most of this also holds true for Wash and dry hands thoroughly prior to collection. -
![IAC 3/11/09 Pharmacy[657] Ch 13, P.1 CHAPTER 13 STERILE](https://docslib.b-cdn.net/cover/7069/iac-3-11-09-pharmacy-657-ch-13-p-1-chapter-13-sterile-417069.webp)
IAC 3/11/09 Pharmacy[657] Ch 13, P.1 CHAPTER 13 STERILE
IAC 3/11/09 Pharmacy[657] Ch 13, p.1 CHAPTER 13 STERILE COMPOUNDING PRACTICES 657—13.1(124,126,155A) Purpose and scope. These rules establish standards and procedures for the preparation, labeling, and distribution of sterile preparations by licensed pharmacies pursuant to a practitioner’s order or prescription; for sterile product quality and characteristics; for personnel training, environmental quality, and equipment standards; and for pharmaceutical care. Sterile compounding differs from nonsterile compounding primarily by requiring the maintenance of sterility when preparations are compounded exclusively with sterile ingredients and components and by requiring the achievement of sterility when preparations are compounded with nonsterile ingredients and components. The standards and procedures outlined in this chapter apply to pharmacy practice when a preparation: 1. Is prepared according to the manufacturer’s labeled instructions and requires other manipulations that expose the original contents to potential contamination; 2. Contains nonsterile ingredients or employs nonsterile components or devices that must be sterilized before administration; or 3. Is a biologic, diagnostic, drug, or nutrient that possesses characteristics of either “1” or “2” above and includes, but is not limited to, the following preparations that are required to be sterile when they are administered to patients: baths and soaks for live organs and tissues, injections (e.g., colloidal dispersions, emulsions, solutions, and suspensions), aqueous bronchial and -

Aseptic Processing White Paper
WHITE PAPER ASEPTIC PROCESSING WHILE MAINTAINING PRODUCT INTEGRITY Published by Marlen A MARLEN WHITE PAPER: Aseptic Processing While Maintaining Product Integrity INTRODUCTION Aseptic processing and packaging is one of the most dynamic areas of food processing. Although invented in the middle of the twentieth century, recently a panel of food technologists and scientists from the Institute of Food Technologists (IFT) with the intention of identifying leading food processing and packaging achievements advised that “Aseptic processing is the most significant food science development in the last 50 years.” The most basic definition of aseptic processing is the independent sterilization of the product followed by filling and sealing (packaging) in sterile containers in a sterile environment. Also known as high-temperature-short-time (HTST) processing, more and more processors are realizing the benefits of aseptic processing and packaging. Therefore, the number of processors utilizing this technology has significantly increased. With the recent advent of the U.S. Food and Drug Administration approval of aseptic processing of food products containing particulate matter, we expect this method of food processing to explode with activity. Marlen pumps facilitate this and fulfill one of the most critical areas of the process. THE PROBLEM In addition to extending the life of shelf stable products, processors must protect heat-sensitive products such as produce, soups, sauces and baby food from microbial degradation. Until recently, continuous thermal aseptic processing solutions using ultra-high temperatures were not approved by the U.S. Food and Drug Administration, forcing processors to use low acid canned food (LACF) methods that compromised the nutritional and organoleptic properties of the food products. -

Rigid Intermediate Bulk Containers: United States
Freedonia Focus Reports US Collection Rigid Intermediate Bulk Containers: United States February 2019 CLICK TO ORDER FULL REPORT BROCHURE www.freedoniafocusreports.com CLICK TO ORDER FULL REPORT Table of Contents 1. Highlights 3 2. Market Environment 4 Historical Trends 4 Key Economic Indicators 5 Competition from Reconditioned & Refurbished Packaging 6 Regulatory Factors 8 3. Segmentation & Forecasts 10 Products 10 Plastic Body 11 Metal Body 12 Other Rigid Intermediate Bulk Containers 13 Markets 14 Chemicals & Pharmaceuticals 15 Food & Beverages 15 Petroleum & Lubricants 16 Hazardous Waste 16 Agricultural & Horticultural Products 16 Plastic & Rubber 17 Other Markets 17 4. Industry Structure 19 Industry Characteristics 19 Market Leaders 20 Mauser Packaging Solutions 20 SCHÜTZ 21 Greif 21 5. About This Report 22 Scope 22 Sources 22 Industry Codes 23 Freedonia Methodology 23 Resources 25 Rigid Intermediate Bulk Containers: United States 1 ©2019 The Freedonia Group. All rights reserved. List of Tables & Figures Figure 1 | Key Trends in the US Rigid Intermediate Bulk Container Market, 2018 – 2023 3 Figure 2 | US Rigid Intermediate Bulk Container Demand Trends, 2008 – 2018 4 Table 1 | Key Indicators for US Rigid Intermediate Bulk Container Demand, 2008 – 2023 (US$ bil) 5 Figure 3 | US Rigid Intermediate Bulk Container Demand by Product, 2008 – 2023 (US$ mil) 10 Table 2 | US Rigid Intermediate Bulk Container Demand by Product, 2008 – 2023 (US$ mil) 10 Figure 4 | US Rigid Intermediate Bulk Container Demand by Product, 2008 – 2023 (%) 12 Figure 5 | US Rigid Intermediate Bulk Container Demand by Market, 2008 – 2023 (US$ mil) 14 Table 3 | US Rigid Intermediate Bulk Container Demand by Market, 2008 – 2023 (US$ mil) 14 Figure 6 | US Rigid Intermediate Bulk Container Demand by Market, 2008 – 2023 (%) 18 Table 4 | Leading Suppliers in the US Rigid Intermediate Bulk Container Market by Product 20 Table 5 | NAICS & SIC Codes Related to Rigid Intermediate Bulk Containers 23 Rigid Intermediate Bulk Containers: United States 2 ©2019 The Freedonia Group. -

Nalgene and Nunc Centrifuge Ware Catalog
Nalgene and Nunc Centrifuge Ware Select the right vessel and spin with confidence Spin with confidence at virtually any scale The process of selecting a centrifuge and rotor can feel like the easy part when faced with choosing the tube or bottle that is the right fit for both the rotor and application. There are several factors to consider when selecting the correct vessel for each application: • Chemical compatibility • Volume • Temperature • Relative centrifugal force (RCF) required • Protocols to be used for loading and sample recovery • Cleaning and autoclaving steps Understanding your requirements before selecting a tube or bottle ensures you make the right choice. Whether your application includes the need for separations, large volume pelleting, protein purification or DNA isolation, the comprehensive selection of Thermo Scientific™ Nalgene™ and Thermo Scientific™ Nunc™ centrifuge ware offers a solution for virtually scales and is available in sizes from 10 mL to 2 L. Such a broad offering means a tube or bottle for many spins – from clinical and bioproduction, to processing bacteria, yeast, tissue, and viruses. Contents Centrifuge tubes 4 Centrifuge bottles 20 Closures and adaptors 31 Resources 36 Simplify performance at every turn with a reliable and safe approach to centrifugation Nalgene Conical-Bottom Centrifuge Tubes polypropylene copolymer Thermo Scientific™ Nalgene™ PPCO conical-bottom centrifuge tubes with molded-in graduations have excellent chemical resistance. Designed for low-speed centrifugation in refrigerated and non-refrigerated centrifuges details • Translucent PPCO is compatible with a wide range of lab reagents • Conical bottoms concentrate pellet in a small area for easy isolation and retrieval • Molded-in graduations last the life of the tube • Last longer than polycarbonate tubes under conditions of repeated Note: Centrifuge tubes must be filled at least 80% for proper performance. -

Thermal Processing Outline
Thermal Processing Outline 1. Classification of Foods based on pH and aw 2. Microbiology 3. Blanching, Pasteurization, ESL (incl. Ultra- Pasteurization), UHT, Hot Fill, Minimal processing 4. Canning Operations 5. Thermal Processing Equipment 6. Kinetics (D & z values) 7. Process Safety (F value) and Product Quality (C value) 8. Process Optimization 9. Shelf Life 10.Time Temperature Integrator (TTI) 1. Classification of Foods Based on pH & aw Classification of Foods based on pH • Low acid: pH ≥ 4.6; Acid: pH < 4.6 (C. botulinum) • More specific classification – Low acid: pH > 5.3 • Red meat, poultry, seafood, milk, corn, peas, lima beans, potatoes, cauliflower – Medium acid: 4.5 < pH < 5.3 • Spaghetti, soups, sauces, asparagus, beets, pumpkin, spinach, green beans, turnip, cabbage – Acid: 3.7 < pH < 4.5 • Tomato, pear, fig, pineapple, apricot, yogurt, white cheese, beer – High acid: pH < 3.7 • Sauerkraut, pickles, berries, citrus, rhubarb, wine, vinegar, plums, currants, apples, strawberries, peaches Classification of Foods Based on mc or aw • High moisture foods (50+% -- 70-99%) – Fruits, vegetables, juices, raw meat, fish • Intermediate moisture foods (15-50%) – Bread, hard cheeses, sausages • Low moisture foods (0-15%) – Dehydrated vegetables, grains, milk powder, dry soup mixes Importance of aw: Honey at 20% mc is shelf stable, while potato at 20% is not 2. Microbiology Classification of Bacteria • Based on Oxygen – Aerobes (Need oxygen for growth) • Microaerophile: Need only small amount of oxygen for growth – Anaerobes • Obligate: -
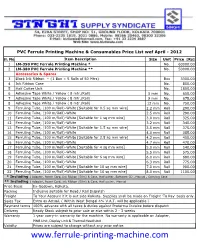
Ferrule Printing Machine & Consumables Price List Wef April - 2012
PVC Ferrule Printing Machine & Consumables Price List wef April - 2012 Sl. No. Item Description Size Unit Price [Rs] 1 LM-390 PVC Ferrule Printing Machine * No. 60000.00 2 LM-380 PVC Ferrule Printing Machine ** No. 50000.00 Accessories & Spares 3 Black Ink Ribbon – {1 Box = 5 Rolls of 50 Mtrs} Box 3300.00 4 Ink Ribbon Case No. 850.00 5 Half Cutter Unit No. 1800.00 6 Adhesive Tape White / Yellow ( 8 mtr./Roll) 5 mm No. 650.00 7 Adhesive Tape White / Yellow ( 8 mtr./Roll) 9 mm No. 675.00 8 Adhesive Tape White / Yellow ( 8 mtr./Roll) 12 mm No. 750.00 9 Ferruling Tube, (100 m/Roll)-White [Suitable for 0.5 sq mm wire] 2.2 mm Roll 290.00 10 Ferruling Tube, (100 m/Roll)-White 2.5 mm Roll 290.00 11 Ferruling Tube, (100 m/Roll)-White [Suitable for 1 sq mm wire] 3.0 mm Roll 325.00 12 Ferruling Tube, (100 m/Roll)-White 3.2 mm Roll 340.00 13 Ferruling Tube, (100 m/Roll)-White [Suitable for 1.5 sq mm wire] 3.5 mm Roll 375.00 14 Ferruling Tube, (100 m/Roll)-White 4.0 mm Roll 385.00 15 Ferruling Tube, (100 m/Roll)-White [Suitable for 2.5 sq mm wire] 4.2 mm Roll 450.00 16 Ferruling Tube, (100 m/Roll)-White 4.7 mm Roll 470.00 17 Ferruling Tube, (100 m/Roll)-White [Suitable for 4 sq mm wire] 5.0 mm Roll 540.00 18 Ferruling Tube, (100 m/Roll)-White 5.5 mm Roll 575.00 19 Ferruling Tube, (100 m/Roll)-White [Suitable for 6 sq mm wire] 6.0 mm Roll 680.00 20 Ferruling Tube, (100 m/Roll)-White 6.3 mm Roll 775.00 21 Ferruling Tube, (100 m/Roll)-White [Suitable for 10 sq mm wire] 7.0 mm Roll 900.00 22 Ferruling Tube, (100 m/Roll)-White [Suitable for 16 sq mm wire] 8.0 mm Roll 1100.00 * Including : Adapter, Power Cord, Ink Ribbon (50m) & Case, Half cutter, Software CD , Manual , Carrying Case ** Including : Adapter, Power Cord, Ink Ribbon (50m) & Case, Half cutter, Manual Price Basis Ex-Godown, Kolkata.