Problem Solving Exercises in Cardiovascular Physiology and Pathophysiology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Blood Vessels: Part A
Chapter 19 The Cardiovascular System: Blood Vessels: Part A Blood Vessels • Delivery system of dynamic structures that begins and ends at heart – Arteries: carry blood away from heart; oxygenated except for pulmonary circulation and umbilical vessels of fetus – Capillaries: contact tissue cells; directly serve cellular needs – Veins: carry blood toward heart Structure of Blood Vessel Walls • Lumen – Central blood-containing space • Three wall layers in arteries and veins – Tunica intima, tunica media, and tunica externa • Capillaries – Endothelium with sparse basal lamina Tunics • Tunica intima – Endothelium lines lumen of all vessels • Continuous with endocardium • Slick surface reduces friction – Subendothelial layer in vessels larger than 1 mm; connective tissue basement membrane Tunics • Tunica media – Smooth muscle and sheets of elastin – Sympathetic vasomotor nerve fibers control vasoconstriction and vasodilation of vessels • Influence blood flow and blood pressure Tunics • Tunica externa (tunica adventitia) – Collagen fibers protect and reinforce; anchor to surrounding structures – Contains nerve fibers, lymphatic vessels – Vasa vasorum of larger vessels nourishes external layer Blood Vessels • Vessels vary in length, diameter, wall thickness, tissue makeup • See figure 19.2 for interaction with lymphatic vessels Arterial System: Elastic Arteries • Large thick-walled arteries with elastin in all three tunics • Aorta and its major branches • Large lumen offers low resistance • Inactive in vasoconstriction • Act as pressure reservoirs—expand -

Pathophysiology of the Cardiovascular System and Neonatal Hypotension
Pathophysiology of the Cardiovascular System and Neonatal Hypotension Sandra L. Shead, RNC-NIC, MSN, CNS, NNP-BC Continuing Nursing Education BSTRACT (CNE) Credit A A total of 2.5 contact Hypotension is common in low birth weight neonates and less common in term newborns and is hours may be earned as CNE credit associated with significant morbidity and mortality. Determining an adequate blood pressure in for reading the articles in this issue neonates remains challenging for the neonatal nurse because of the lack of agreed-upon norms. Values identified as CNE and for completing an online posttest and evaluation. for determining norms for blood pressure at varying gestational and postnatal ages are based on To be successful the learner must empirical data. Understanding cardiovascular pathophysiology, potential causes of hypotension, and obtain a grade of at least 80% on assessment of adequate perfusion in the neonatal population is important and can assist the neonatal the test. Test expires three (3) years from publication date. Disclosure: nurse in the evaluation of effective blood pressure. This article reviews cardiovascular pathophysiology The author/planning committee as it relates to blood pressure and discusses potential causes of hypotension in the term and preterm has no relevant financial interest or affiliations with any commercial neonate. Variation in management of hypotension across centers is discussed. Underlying causes and interests related to the subjects pathophysiology of hypotension in the neonate are described. discussed within this article. No commercial support or sponsorship was provided for this educational Keywords: neonatal hypotension; definition; physiology; cardiovascular system; preterm; management; activity. ANN/ANCC does not assessment; cerebral; autoregulation; blood pressure endorse any commercial products discussed/displayed in conjunction with this educational activity. -

Arterial System Lecture Block 10 Vascular Structure/Function
Arterial System Lecture Block 10 Arterial System Bioengineering 6000 CV Physiology Vascular Structure/Function Arterial System Bioengineering 6000 CV Physiology Functional Overview Arterial System Bioengineering 6000 CV Physiology Vessel Structure Aorta Artery Vein Vena Cava Arteriole Capillary Venule Diameter 25 mm 4 mm 5 mm 30 mm 30 µm 8 µm 20 µm Wall 2 mm 1 mm 0.5 mm 1.5 mm 6 µm 0.5 µm 1 µm thickness Endothelium Elastic tissue Smooth Muscle Fibrous Tissue Arterial System Bioengineering 6000 CV Physiology Aortic Compliance • Factors: – age 20--24 yrs – athersclerosis 300 • Effects – more pulsatile flow 200 dV 30--40 yrs C = – more cardiac work dP 50-60 yrs – not hypertension Laplace’s Law 100 70--75 yrs (thin-walled cylinder): [%] Volume Blood T = wall tension P = pressure T = Pr r = radius For thick wall cylinder 100 150 200 P = pressure Pr Pressure [mm Hg] σ = wall stress r = radius σ = Tension Wall Stress w = wall thickness w [dyne/cm] [dyne/cm2] Aorta 2 x 105 10 x 105 Capillary 15-70 1.5 x 105 Arterial System Bioengineering 6000 CV Physiology Arterial Hydraulic Filter Arterial System Bioengineering 6000 CV Physiology Arterial System as Hydraulic Filter Arterial Cardiac Pressure • Pulsatile --> Output t Physiological smooth flow t Ideal • Cardiac energy conversion Cardiac • Reduces total Output Arterial cardiac work Pressure t Pulsatile t Challenge Cardiac Output Arterial Pressure t Filtered t Reality Arterial System Bioengineering 6000 CV Physiology Elastic Recoil in Arteries Arterial System Bioengineering 6000 CV Physiology Effects of Vascular Resistance and Compliance Arterial System Bioengineering 6000 CV Physiology Cardiac Output vs. -

Vascular Peripheral Resistance and Compliance in the Lobster Homarus Americanus
The Journal of Experimental Biology 200, 477–485 (1997) 477 Printed in Great Britain © The Company of Biologists Limited 1997 JEB0437 VASCULAR PERIPHERAL RESISTANCE AND COMPLIANCE IN THE LOBSTER HOMARUS AMERICANUS JERREL L. WILKENS*,1, GLEN W. DAVIDSON†,1 AND MICHAEL J. CAVEY1,2 1Department of Biological Sciences, The University of Calgary, 2500 University Drive NW, Calgary, Alberta, Canada T2N 1N4 and 2Department of Anatomy, The University of Calgary, 3330 Hospital Drive NW, Calgary, Alberta, Canada T2N 4N1 Accepted 24 October 1996 Summary The peripheral resistance to flow through each arterial become stiffer at pressures greater than peak systolic bed (in actuality, the entire pathway from the heart back pressure and at radii greater than twice the unpressurized to the pericardial sinus) and the mechanical properties of radius. The dorsal abdominal artery possesses striated the seven arteries leaving the lobster heart are measured muscle in the lateral walls. This artery remains compliant and compared. Resistance is inversely proportional to over the entire range of hemolymph pressures expected in artery radius and, for each pathway, the resistance falls lobsters. These trends are illustrated when the non-linearly as flow rate increases. The resistance of the incremental modulus of elasticity is compared among hepatic arterial system is lower than that predicted on the arteries. All arteries should function as Windkessels to basis of its radius. Body-part posture and movement may damp the pulsatile pressures and flows generated by the affect the resistance to perfusion of that region. The total heart. The dorsal abdominal artery may also actively vascular resistance placed on the heart when each artery regulate its flow. -

Pathophysiology of the Right Ventricle And€Of the Pulmonary Circulation In
SERIES | WORLD SYMPOSIUM ON PULMONARY HYPERTENSION Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: an update Anton Vonk Noordegraaf1, Kelly Marie Chin2, François Haddad3, Paul M. Hassoun4, Anna R. Hemnes5, Susan Roberta Hopkins6, Steven Mark Kawut7, David Langleben8, Joost Lumens9,10 and Robert Naeije11,12 Number 3 in the series “Proceedings of the 6th World Symposium on Pulmonary Hypertension” Edited by N. Galiè, V.V. McLaughlin, L.J. Rubin and G. Simonneau Affiliations: 1Amsterdam UMC, Vrije Universiteit Amsterdam, Pulmonary Medicine, Amsterdam Cardiovascular Sciences, Amsterdam, The Netherlands. 2Division of Pulmonary and Critical Care Medicine, University of Texas Southwestern, Dallas, TX, USA. 3Division of Cardiovascular Medicine, Stanford University and Stanford Cardiovascular Institute, Palo Alto, CA, USA. 4Division of Pulmonary and Critical Care Medicine, Johns Hopkins University, Baltimore, MD, USA. 5Division of Allergy, Pulmonary and Critical Care Medicine, Vanderbilt University Medical Center, Nashville, TN, USA. 6Dept of Medicine, University of California, San Diego, La Jolla, CA, USA. 7Penn Cardiovascular Institute, Dept of Medicine, and Center for Clinical Epidemiology and Biostatistics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA. 8Center for Pulmonary Vascular Disease, Cardiology Division, Jewish General Hospital and McGill University, Montreal, QC, Canada. 9Maastricht University Medical Center, CARIM School for Cardiovascular Diseases, Maastricht, The Netherlands. 10Université de Bordeaux, LIRYC (L’Institut de Rythmologie et Modélisation Cardiaque), Bordeaux, France. 11Dept of Cardiology, Erasme University Hospital, Brussels, Belgium. 12Laboratory of Cardiorespiratory Exercise Physiology, Faculty of Motor Sciences, Université Libre de Bruxelles, Brussels, Belgium. Correspondence: Anton Vonk Noordegraaf, Amsterdam UMC, Vrije Universiteit Amsterdam, Pulmonary Medicine, Amsterdam Cardiovascular Sciences, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands. -

Effects of Vasodilation and Arterial Resistance on Cardiac Output Aliya Siddiqui Department of Biotechnology, Chaitanya P.G
& Experim l e ca n i t in a l l C Aliya, J Clinic Experiment Cardiol 2011, 2:11 C f a Journal of Clinical & Experimental o r d l DOI: 10.4172/2155-9880.1000170 i a o n l o r g u y o J Cardiology ISSN: 2155-9880 Review Article Open Access Effects of Vasodilation and Arterial Resistance on Cardiac Output Aliya Siddiqui Department of Biotechnology, Chaitanya P.G. College, Kakatiya University, Warangal, India Abstract Heart is one of the most important organs present in human body which pumps blood throughout the body using blood vessels. With each heartbeat, blood is sent throughout the body, carrying oxygen and nutrients to all the cells in body. The cardiac cycle is the sequence of events that occurs when the heart beats. Blood pressure is maximum during systole, when the heart is pushing and minimum during diastole, when the heart is relaxed. Vasodilation caused by relaxation of smooth muscle cells in arteries causes an increase in blood flow. When blood vessels dilate, the blood flow is increased due to a decrease in vascular resistance. Therefore, dilation of arteries and arterioles leads to an immediate decrease in arterial blood pressure and heart rate. Cardiac output is the amount of blood ejected by the left ventricle in one minute. Cardiac output (CO) is the volume of blood being pumped by the heart, by left ventricle in the time interval of one minute. The effects of vasodilation, how the blood quantity increases and decreases along with the blood flow and the arterial blood flow and resistance on cardiac output is discussed in this reviewArticle. -

Determination of Cardiac Output by Equating Venous Return Curves with Cardiac Response Curves
Determination of Cardiac Output By Equating Ve- nous Return Curves With Cardiac Response Curves1 ARTHUR C. GUYTQN From the Department of Physiology and Biophysics, School of Medicine, University of Mississippi, University, Mississippi HE CONCEPT that the heart responds with increasing cardiac output when there occurs increasing venous return was popularized by Starling and, in- deed, has come to be known as Starling’s law. There are many different forms in which Starling’s law can be expressed,including the relationship of cardiac output to right atria1 pressure, the relationship of cardiac output to the degree of distention of the right ventricle at the end of diastole, the relationship of cardiac work to right atria1 pressure or right ventricular distention, the relationship of left ventricular work to right atria1 pressure or right ventricular distention, etc. For the determina- tion of cardiac output, the form of Starling’s law which will be used in the present discussion is the relationship of cardiac output to mean right atria1 pressure, and this type of cruve will be called the “cardiac responsecurve” to right atria1 pressure. It is well known that many factors in the peripheral circulatory system combine together to determine the rate of venous return to the heart. These include the quantity of blood available, the degree of vascular resistance in various parts of the peripheral circulatory system, and the back pressure from the right atrium. It is with these factors that this paper is especially concerned, and it is hoped that this presentation will demonstrate how cardiac output is determined by equating the peripheral circulatory factors with the cardiac responsecurves. -
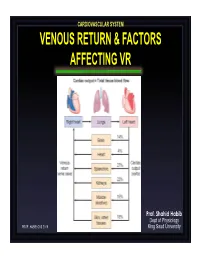
Venous Return & Factors Affecting Vr
CARDIOVASCULAR SYSTEM VENOUS RETURN & FACTORS AFFECTING VR Prof. Shahid Habib Dept of Physiology PROF. HABIB CVS 2019 King Saud University OBJECTIVES At the end of the lecture you should be able to ….. Discuss functions of the veins as blood reservoirs. Describe measurement of central venous pressure (CVP) and state its physiological and clinical significance. State determinants of venous return and explain how they influence venous return. Define mean systemic filling pressure, give its normal value and describe the factors which affect it. Explain the effect of gravity on venous pressure and explain pathophysiology of varicose veins. Describe vascular and cardiac function curves under physiological and pathophysiological conditions. DISTRIBUTION OF BLOOD Capacitance Vessels Veins are blood reservoirs When the body is at rest and many of the capillaries are closed, the capacity of the venous reservoir is increased as extra blood bypasses the capillaries and enters the veins. When this extra volume of blood stretches the veins, the blood moves forward through the veins more slowly because the total cross sectional area of the veins has increased as a result of the stretching. Therefore, blood spends more time in the veins. When the stored blood is needed, such as during exercise, extrinsic factors reduce the capacity of the venous reservoir and drive the extra blood from the veins to the heart so that it can be pumped to the tissues. Rt. Lt. At At Right Left ven ven Large veins Large arteries CAPACITANCE VESSELS EXCHANGE VESSELS RESISTANCE VESSELS Venules Capillaries Arterioles Vascular circuit • all 3 layers are present, but thinner than in •In varicose veins, arteries of corresponding size (external blood pools because diameter). -

Valve Replacement Alexander Zezulkal, John Mackinnon2 and D.G
Postgrad Med J: first published as 10.1136/pgmj.68.797.180 on 1 March 1992. Downloaded from Postgrad Med J (1992) 68, 180 - 185 i) The Fellowship of Postgraduate Medicine, 1992 Hypertension in aortic valve disease and its response to valve replacement Alexander Zezulkal, John Mackinnon2 and D.G. Beevers' 'University Department ofMedicine and 2Department ofCardiology, Dudley Road Hospital, Birmingham BT18 7QH, UK Summary: We have investigated the prevalence ofhypertension and the response ofblood pressure to operation in 87 patients with lone aortic valve disease who underwent aortic valve replacement. In patients with aortic stenosis alone 26% were hypertensive pre-operatively (age and sex adjusted blood pressure > 160 systolic and or > 95 mmHg diastolic) and 24% were hypertensive post-operatively. In those with aortic regurgitation alone, hypertension was present in 65% before and 57% after valve replacement using the same criterion. For combined stenosis and regurgitation, the prevalence was 54% and 62%, respectively. The post-operative increase in systolic pressure in patients with aortic stenosis occurred mainly in those with a history of left ventricular failure. In those with aortic regurgitation or combined stenosis with regurgitation, diastolic pressure rose after valve replacement resulting in a prevalence of diastolic hypertension of 44% and 35%, respectively. Blood pressure changes were not predicted by the type of valve inserted nor its size. Our data show that despite severe symptomatic aortic valve disease, systolic hypertension was common in aortic stenosis and diastolic hypertension was found in aortic regurgitation. This underlines the by copyright. importance of blood pressure monitoring in patients following aortic valve replacement. -

Time-Varying Elastance and Left Ventricular Aortic Coupling Keith R
Walley Critical Care (2016) 20:270 DOI 10.1186/s13054-016-1439-6 REVIEW Open Access Left ventricular function: time-varying elastance and left ventricular aortic coupling Keith R. Walley Abstract heart must have special characteristics that allow it to respond appropriately and deliver necessary blood flow Many aspects of left ventricular function are explained and oxygen, even though flow is regulated from outside by considering ventricular pressure–volume characteristics. the heart. Contractility is best measured by the slope, Emax, of the To understand these special cardiac characteristics we end-systolic pressure–volume relationship. Ventricular start with ventricular function curves and show how systole is usefully characterized by a time-varying these curves are generated by underlying ventricular elastance (ΔP/ΔV). An extended area, the pressure– pressure–volume characteristics. Understanding ventricu- volume area, subtended by the ventricular pressure– lar function from a pressure–volume perspective leads to volume loop (useful mechanical work) and the ESPVR consideration of concepts such as time-varying ventricular (energy expended without mechanical work), is linearly elastance and the connection between the work of the related to myocardial oxygen consumption per beat. heart during a cardiac cycle and myocardial oxygen con- For energetically efficient systolic ejection ventricular sumption. Connection of the heart to the arterial circula- elastance should be, and is, matched to aortic elastance. tion is then considered. Diastole and the connection of Without matching, the fraction of energy expended the heart to the venous circulation is considered in an ab- without mechanical work increases and energy is lost breviated form as these relationships, which define how during ejection across the aortic valve. -

04. the Cardiac Cycle/Wiggers Diagram
Part I Anaesthesia Refresher Course – 2018 4 University of Cape Town The Cardiac Cycle The “Wiggers diagram” Prof. Justiaan Swanevelder Dept of Anaesthesia & Perioperative Medicine University of Cape Town Each cardiac cycle consists of a period of relaxation (diastole) followed by ventricular contraction (systole). During diastole the ventricles are relaxed to allow filling. In systole the right and left ventricles contract, ejecting blood into the pulmonary and systemic circulations respectively. Ventricles The left ventricle pumps blood into the systemic circulation via the aorta. The systemic vascular resistance (SVR) is 5–7 times greater than the pulmonary vascular resistance (PVR). This makes it a high-pressure system (compared with the pulmonary vascular system), which requires a greater mechanical power output from the left ventricle (LV). The free wall of the LV and the interventricular septum form the bulk of the muscle mass in the heart. A normal LV can develop intraventricular pressures up to 300 mmHg. Coronary perfusion to the LV occurs mainly in diastole, when the myocardium is relaxed. The right ventricle receives blood from the venae cavae and coronary circulation, and pumps it via the pulmonary vasculature into the LV. Since PVR is a fraction of SVR, pulmonary arterial pressures are relatively low and the wall thickness of the right ventricle (RV) is much less than that of the LV. The RV thus resembles a passive conduit rather than a pump. Coronary perfusion to the RV occurs continuously during systole and diastole because of the low intraventricular and intramural pressures. In spite of the anatomical differences, the mechanical behaviour of the RV and LV is very similar. -

Pulmonary Vascular Resistance and Compliance Relationship in Pulmonary Hypertension
SERIES PHYSIOLOGY IN RESPIRATORY MEDICINE Pulmonary vascular resistance and compliance relationship in pulmonary hypertension Denis Chemla1,2, Edmund M.T. Lau1,2,3, Yves Papelier2, Pierre Attal4 and Philippe Hervé5 Number 10 in the series “Physiology in respiratory medicine” Edited by R. Naeije, D. Chemla, A. Vonk-Noordegraaf and A.T. Dinh-Xuan Affiliations: 1Univ. Paris-Sud, Faculté de Médecine, Inserm U_999, Le Kremlin Bicêtre, France. 2AP-HP, Services des Explorations Fonctionnelles, Hôpital de Bicêtre, Le Kremlin Bicêtre, France. 3Dept of Respiratory Medicine, Royal Prince Alfred Hospital, University of Sydney, Camperdown, Australia. 4Dept of Otolaryngology-Head and Neck Surgery, Shaare-Zedek Medical Center and Hebrew University Medical School, Jerusalem, Israel. 5Centre Chirurgical Marie Lannelongue, Le Plessis-Robinson, France. Correspondence: Denis Chemla, Service des Explorations Fonctionnelles - Broca 7, Hôpital de Bicêtre, 78 rue du Général Leclerc, 94 275 Le Kremlin Bicêtre, France. E-mail: [email protected] ABSTRACT Right ventricular adaptation to the increased pulmonary arterial load is a key determinant of outcomes in pulmonary hypertension (PH). Pulmonary vascular resistance (PVR) and total arterial compliance (C) quantify resistive and elastic properties of pulmonary arteries that modulate the steady and pulsatile components of pulmonary arterial load, respectively. PVR is commonly calculated as transpulmonary pressure gradient over pulmonary flow and total arterial compliance as stroke volume over pulmonary arterial pulse pressure (SV/PApp). Assuming that there is an inverse, hyperbolic relationship between PVR and C, recent studies have popularised the concept that their product (RC-time of the pulmonary circulation, in seconds) is “constant” in health and diseases. However, emerging evidence suggests that this concept should be challenged, with shortened RC-times documented in post-capillary PH and normotensive subjects.