Unit Conversions Tool
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Calculating Growing Degree Days by Jim Nugent District Horticulturist Michigan State University
Calculating Growing Degree Days By Jim Nugent District Horticulturist Michigan State University Are you calculating degree day accumulations and finding your values don't match the values being reported by MSU or your neighbor's electronic data collection unit? There is a logical reason! Three different methods are used to calculate degree days; i.e., 1) Averaging Method; 2) Baskerville-Emin (BE) Method; and 3) Electronic Real-time Data Collection. All methods attempt to calculate the heat accumulation above a minimum threshold temperature, often referred to the base temperature. Once both the daily maximum and minimum temperatures get above the minimum threshold temperature, (i.e., base temperature of 42degrees, 50degrees or whatever other base temperature of interest) then all methods are fairly comparable. However, differences do occur and these are accentuated in an exceptionally long period of cool spring temperatures, such as we experienced this year. Let me briefly explain: 1. Averaging Method: Easy to calculate Degree Days(DD) = Average daily temp. - Base Temp. = (max. + min.) / 2 - Base temp. If answer is negative, assume 0. Example: Calculate DD Base 50 given 65 degrees max. and 40 degrees min. Avg. = (65 + 40) / 2 = 52.5 degrees DD base 50 = 52.5 - 50 = 2.5 degrees. But look what happens given a maximum of 60 degrees and a minimum of 35 degrees: Avg. = (60 + 35) / 2 = 47.5 DD base 50 = 47.5 - 50 = -2.5 = 0 degrees. The maximum temperature was higher than the base of 50° , but no degree days were accumulated. A grower called about the first of June reporting only about 40% of the DD50 that we have recorded. -

Lord Kelvin and the Age of the Earth.Pdf
ME201/MTH281/ME400/CHE400 Lord Kelvin and the Age of the Earth Lord Kelvin (1824 - 1907) 1. About Lord Kelvin Lord Kelvin was born William Thomson in Belfast Ireland in 1824. He attended Glasgow University from the age of 10, and later took his BA at Cambridge. He was appointed Professor of Natural Philosophy at Glasgow in 1846, a position he retained the rest of his life. He worked on a broad range of topics in physics, including thermody- namics, electricity and magnetism, hydrodynamics, atomic physics, and earth science. He also had a strong interest in practical problems, and in 1866 he was knighted for his work on the transtlantic cable. In 1892 he became Baron Kelvin, and this name survives as the name of the absolute temperature scale which he proposed in 1848. During his long career, Kelvin published more than 600 papers. He was elected to the Royal Society in 1851, and served as president of that organization from 1890 to 1895. The information in this section and the picture above were taken from a very useful web site called the MacTu- tor History of Mathematics Archive, sponsored by St. Andrews University. The web address is http://www-history.mcs.st-and.ac.uk/~history/ 2 kelvin.nb 2. The Age of the Earth The earth shows it age in many ways. Some techniques for estimating this age require us to observe the present state of a time-dependent process, and from that observation infer the time at which the process started. If we believe that the process started when the earth was formed, we get an estimate of the earth's age. -

Having Regard to the Opinion of the European Chapter 1 of the Annex Binding Within Five Years of Parliament1 ; the Date of Entry Into Force of This Directive
878 Official Journal of the European Communities 29.10.71 Official Journal of the European Communities No L 243/29 COUNCIL DIRECTIVE of 18 October 1971 on the approximation of the laws of the Member States relating to units of measurement (71/354/EEC ) THE COUNCIL OF THE EUROPEAN COMMUNITIES, particular their names, symbols and use are not identical in the Member countries ; Having regard to the Treaty establishing the European Economic Community, and in particular HAS ADOPTED THIS DIRECTIVE : Article 100 thereof; Article 1 Having regard to the proposal from the Commission ; 1 . Member States shall make the provisions of Having regard to the Opinion of the European Chapter 1 of the Annex binding within five years of Parliament1 ; the date of entry into force of this Directive. 2 . Member States shall, with effect from 31 Having regard to the Opinion of the Economic and December 1977 at the latest, prohibit the use of the Social Committee2; units of measurement listed in Chapter III of the Annex. Whereas - the laws which regulate the use of units of measurement in the Member States differ from one 3 . The units of measurement temporarily" retained Member State to another and therefore hinder trade ; in accordance with the provisions of Chapter II or whereas application of the rules relating to measuring Chapter III of the Annex may not be brought into instruments is closely linked to the use of units of compulsory use by the Member States where they ' are measurement in the metrological system ; whereas, in not authorised at the date when this Directive enters into force . -

Angles ANGLE Topics • Coterminal Angles • Defintion of an Angle
Angles ANGLE Topics • Coterminal Angles • Defintion of an angle • Decimal degrees to degrees, minutes, seconds by hand using the TI-82 or TI-83 Plus • Degrees, seconds, minutes changed to decimal degree by hand using the TI-82 or TI-83 Plus • Standard position of an angle • Positive and Negative angles ___________________________________________________________________________ Definition: Angle An angle is created when a half-ray (the initial side of the angle) is drawn out of a single point (the vertex of the angle) and the ray is rotated around the point to another location (becoming the terminal side of the angle). An angle is created when a half-ray (initial side of angle) A: vertex point of angle is drawn out of a single point (vertex) AB: Initial side of angle. and the ray is rotated around the point to AC: Terminal side of angle another location (becoming the terminal side of the angle). Hence angle A is created (also called angle BAC) STANDARD POSITION An angle is in "standard position" when the vertex is at the origin and the initial side of the angle is along the positive x-axis. Recall: polynomials in algebra have a standard form (all the terms have to be listed with the term having the highest exponent first). In trigonometry, there is a standard position for angles. In this way, we are all talking about the same thing and are not trying to guess if your math solution and my math solution are the same. Not standard position. Not standard position. This IS standard position. Initial side not along Initial side along negative Initial side IS along the positive x-axis. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Applications of Geometry and Trigonometry
P1: FXS/ABE P2: FXS 9780521740517c14.xml CUAU031-EVANS September 6, 2008 13:36 CHAPTER 14 MODULE 2 Applications of geometry and trigonometry How do we apply trigonometric techniques to problems involving angles of depression and elevation? How do we apply trigonometric techniques to problems involving bearings? How do we apply trigonometric techniques to three-dimensional problems? How do we draw and interpret contour maps? How do we draw and interpret scale drawings? 14.1 Angles of elevation and depression, bearings, and triangulation Angles of elevation and depression The angle of elevation is the angle The angle of depression is the angle between the horizontal and a between the horizontal and a direction below direction above the horizontal. the horizontal. eye level line of sight angle of depression line of sight eye level cliff angle of elevation SAMPLEboat Cambridge University Press • Uncorrected Sample pages • 978-0-521-61328-6 • 2008 © Jones, Evans, Lipson TI-Nspire & Casio ClassPad material in collaboration with Brown410 and McMenamin P1: FXS/ABE P2: FXS 9780521740517c14.xml CUAU031-EVANS September 6, 2008 13:36 Chapter 14 — Applications of geometry and trigonometry 411 Bearings The three-figure bearing (or compass bearing) is the direction measured clockwise from north. The bearing of A from O is 030◦. The bearing of C from O is 210◦. The bearing of B from O is 120◦. The bearing of D from O is 330◦. N D N A 30° 330° E 120° E W O 210° W O B C S S Example 1 Angle of depression The pilot of a helicopter flying at 400 m observes a small boat at an angle of depression of 1.2◦. -

1. What Is the Radian Measure of an Angle Whose Degree Measure Is 72◦ 180◦ 72◦ = Πradians Xradians Solving for X 72Π X = Radians 180 2Π X = Radians 5 Answer: B
1. What is the radian measure of an angle whose degree measure is 72◦ 180◦ 72◦ = πradians xradians Solving for x 72π x = radians 180 2π x = radians 5 Answer: B 2. What is the length of AC? Using Pythagorean Theorem: 162 + b2 = 202 256 + b2 = 400 b2 = 144 b = 12 Answer: E 3. one solution to z2 + 64 = 0 is z2 + 64 = 0 z2 = −64 p p z2 = −64 Since there is a negative under the radical, we get imaginary roots. Thus z = −8i or z = +8i Answer: A 4. Simplifying: p 36x10y12 − 36y12 = p36y12 (x10 − 1) p = 36y12p(x10 − 1) p = 6y6 x10 − 1 Answer: C 1 5. Simplifying: 1 1 1 1 1 −3 9 6 3 −3 9 6 27a b c = 27 3 a 3 b 3 c 3 = 3a−1b3c2 3b3c2 = a Answer: C 6. Solving for x: p 8 + x + 14 = 12 p x + 14 = 12 − 8 p x + 14 = 4 p 2 x + 14 = 42 x + 14 = 16 x = 16 − 14 x = 2 Answer: C 7. Simplify x2 − 9 (x + 1)2 2x − 6 × ÷ x2 − 1 (2x + 3)(x + 3) 1 − x Rewrite as a multiplication. x2 − 9 (x + 1)2 1 − x × x2 − 1 (2x + 3)(x + 3) 2x − 6 Simplify each polynomial (x − 3)(x + 3) (x + 1)(x + 1) 1 − x × (x − 1)(x + 1) (2x + 3)(x + 3) 2(x − 3) Canceling like terms (x + 1) − 2(2x + 3) Answer: C 2 8. Simplifying: 1 11 x−5 11 + 2 2 + 2 x−5 (x−5) = (x−5) (x−5) x + 1 x + 1 x−5+11 2 = (x−5) x + 1 x+6 2 = (x−5) x + 1 x + 6 = (x − 5)2(x + 1) Answer: B 9. -

4.1 – Radian and Degree Measure
4.1 { Radian and Degree Measure Accelerated Pre-Calculus Mr. Niedert Accelerated Pre-Calculus 4.1 { Radian and Degree Measure Mr. Niedert 1 / 27 2 Radian Measure 3 Degree Measure 4 Applications 4.1 { Radian and Degree Measure 1 Angles Accelerated Pre-Calculus 4.1 { Radian and Degree Measure Mr. Niedert 2 / 27 3 Degree Measure 4 Applications 4.1 { Radian and Degree Measure 1 Angles 2 Radian Measure Accelerated Pre-Calculus 4.1 { Radian and Degree Measure Mr. Niedert 2 / 27 4 Applications 4.1 { Radian and Degree Measure 1 Angles 2 Radian Measure 3 Degree Measure Accelerated Pre-Calculus 4.1 { Radian and Degree Measure Mr. Niedert 2 / 27 4.1 { Radian and Degree Measure 1 Angles 2 Radian Measure 3 Degree Measure 4 Applications Accelerated Pre-Calculus 4.1 { Radian and Degree Measure Mr. Niedert 2 / 27 The starting position of the ray is the initial side of the angle. The position after the rotation is the terminal side of the angle. The vertex of the angle is the endpoint of the ray. Angles An angle is determine by rotating a ray about it endpoint. Accelerated Pre-Calculus 4.1 { Radian and Degree Measure Mr. Niedert 3 / 27 The position after the rotation is the terminal side of the angle. The vertex of the angle is the endpoint of the ray. Angles An angle is determine by rotating a ray about it endpoint. The starting position of the ray is the initial side of the angle. Accelerated Pre-Calculus 4.1 { Radian and Degree Measure Mr. Niedert 3 / 27 The vertex of the angle is the endpoint of the ray. -
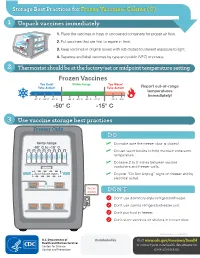
Storage Best Practices for Frozen Vaccines-Celsius
Storage Best Practices for Frozen Vaccines–Celsius (C) 1 Unpack vaccines immediately 1. Place the vaccines in trays or uncovered containers for proper air flow. 2. Put vaccines that are first to expire in front. HEP A - VFC 3. Keep vaccines in original boxes with lids closed to prevent exposure to light. 4. Separate and label vaccines by type and public (VFC) or private. 2 Thermostat should be at the factory-set or midpoint temperature setting Frozen Vaccines Too Cold! Within Range Too Warm! Take Action! Take Action! Report out-of-range temperatures immediately! -57° C -54° C -51° C -46° C -43° C -40° C -37° C -12° C -9°C -50° C -15° C 3 Use vaccine storage best practices Freezer Only DO temp range ✓ Do make sure the freezer door is closed! -50° C to -15° C ✓ Do use water bottles to help maintain consistent temperature. ✓ Do leave 2 to 3 inches between vaccine containers and freezer walls. don’t block vents ✓ Do post “Do Not Unplug” signs on freezer and by electrical outlet. do not unplug DON’T Don’t use dormitory-style refrigerator/freezer. Don’t use combo refrigerator/freezer unit. Don’t put food in freezer. Don’t store vaccines on shelves in freezer door. CS243541-D Revision %FDFNCFS 20 Distributed by Visit www.cdc.gov/vaccines/SandH or contact your state health department for more information. Test Your Knowledge 1 Which of the following units is the best for storing frozen vaccines? Freezer Freezer Freezer Freezer A. Full-size B. Full-size C. -

UNITS This Appendix Explains Some of the Abbreviations1•2 Used For
APPENDIX: UNITS This appendix explains some of the abbreviations1•2 used for units in this book and gives conversion factors to SI units and atomic units: length 1 a0 = 1 bohr = 0.5291771 X 10-10 m 1 A= 1 Angstrom= lo-10 m = 1.889727 ao mass 1 me = 1 atomic unit of mass = mass of an electron 9.109534 X 10-31 kg = 5.485803 X 10-4 U 1 u 1 universal atomic mass unit = one twelfth the mass of a 12c atom 1.6605655 x lo-27 kg = 1822.887 me time 1 t Eh 1 = 1 atomic unit of time = 2.418884 x l0-17 s 1 s = 1 second = 4.134137 x 1016 t/Eh temperature 1 K = 1 Kelvin amount of substance 1 mol = 1 mole 6.022045 x 1023 atoms, molecules, or formula units energy 1 cm-1 = 1 wavenumber 1 kayser 1.986477 x lo-23 J 4.556335 x 10-6 Eh 857 858 APPENDIX: UNITS 1 kcal/mol = 1 kilocalorie per mole 4.184 kJ/mol = 1.593601 x 10-3 Eh 1 eV 1 electron volt = 1.602189 x lo-19 J 3.674902 X 10-2 Eh 1 Eh 1 hartree = 4.359814 x lo-18 J Since so many different energy units are used in the book, it is helpful to have a conversion table. Such a table was calculated from the recommended values of Cohen and Taylor3 for the physical censtants and is given in Table 1. REFERENCES 1. "Standard for Metric Practice", American Society for Testing and Materials, Philadelphia (1976). -

Relationships of the SI Derived Units with Special Names and Symbols and the SI Base Units
Relationships of the SI derived units with special names and symbols and the SI base units Derived units SI BASE UNITS without special SI DERIVED UNITS WITH SPECIAL NAMES AND SYMBOLS names Solid lines indicate multiplication, broken lines indicate division kilogram kg newton (kg·m/s2) pascal (N/m2) gray (J/kg) sievert (J/kg) 3 N Pa Gy Sv MASS m FORCE PRESSURE, ABSORBED DOSE VOLUME STRESS DOSE EQUIVALENT meter m 2 m joule (N·m) watt (J/s) becquerel (1/s) hertz (1/s) LENGTH J W Bq Hz AREA ENERGY, WORK, POWER, ACTIVITY FREQUENCY second QUANTITY OF HEAT HEAT FLOW RATE (OF A RADIONUCLIDE) s m/s TIME VELOCITY katal (mol/s) weber (V·s) henry (Wb/A) tesla (Wb/m2) kat Wb H T 2 mole m/s CATALYTIC MAGNETIC INDUCTANCE MAGNETIC mol ACTIVITY FLUX FLUX DENSITY ACCELERATION AMOUNT OF SUBSTANCE coulomb (A·s) volt (W/A) C V ampere A ELECTRIC POTENTIAL, CHARGE ELECTROMOTIVE ELECTRIC CURRENT FORCE degree (K) farad (C/V) ohm (V/A) siemens (1/W) kelvin Celsius °C F W S K CELSIUS CAPACITANCE RESISTANCE CONDUCTANCE THERMODYNAMIC TEMPERATURE TEMPERATURE t/°C = T /K – 273.15 candela 2 steradian radian cd lux (lm/m ) lumen (cd·sr) 2 2 (m/m = 1) lx lm sr (m /m = 1) rad LUMINOUS INTENSITY ILLUMINANCE LUMINOUS SOLID ANGLE PLANE ANGLE FLUX The diagram above shows graphically how the 22 SI derived units with special names and symbols are related to the seven SI base units. In the first column, the symbols of the SI base units are shown in rectangles, with the name of the unit shown toward the upper left of the rectangle and the name of the associated base quantity shown in italic type below the rectangle. -

The Kelvin and Temperature Measurements
Volume 106, Number 1, January–February 2001 Journal of Research of the National Institute of Standards and Technology [J. Res. Natl. Inst. Stand. Technol. 106, 105–149 (2001)] The Kelvin and Temperature Measurements Volume 106 Number 1 January–February 2001 B. W. Mangum, G. T. Furukawa, The International Temperature Scale of are available to the thermometry commu- K. G. Kreider, C. W. Meyer, D. C. 1990 (ITS-90) is defined from 0.65 K nity are described. Part II of the paper Ripple, G. F. Strouse, W. L. Tew, upwards to the highest temperature measur- describes the realization of temperature able by spectral radiation thermometry, above 1234.93 K for which the ITS-90 is M. R. Moldover, B. Carol Johnson, the radiation thermometry being based on defined in terms of the calibration of spec- H. W. Yoon, C. E. Gibson, and the Planck radiation law. When it was troradiometers using reference blackbody R. D. Saunders developed, the ITS-90 represented thermo- sources that are at the temperature of the dynamic temperatures as closely as pos- equilibrium liquid-solid phase transition National Institute of Standards and sible. Part I of this paper describes the real- of pure silver, gold, or copper. The realiza- Technology, ization of contact thermometry up to tion of temperature from absolute spec- 1234.93 K, the temperature range in which tral or total radiometry over the tempera- Gaithersburg, MD 20899-0001 the ITS-90 is defined in terms of calibra- ture range from about 60 K to 3000 K is [email protected] tion of thermometers at 15 fixed points and also described.