Molecular Properties of a Fermented Manure Preparation Used As Field Spray in Biodynamic Agriculture
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LG Electronics Inc. LG Android 8 Devices (G7 Thinq, V35 Thinq, V30, G6) (MDFPP31/WLANCEP10) Security Target
LG Electronics Inc. LG Android 8 devices (G7 ThinQ, V35 ThinQ, V30, G6) (MDFPP31/WLANCEP10) Security Target Version 0.6 2018/11/26 Prepared for: LG Electronics Inc. 20 Yoido-dong, Youngdungpogu, Seoul 152-721, Korea Prepared By: www.gossamersec.com LG Electronics Inc. LG Android 8 devices (G7 ThinQ, V35 ThinQ, V30, G6) Version 0.6, 2018/11/26 (MDFPP31/WLANCEP10) Security Target 1. SECURITY TARGET INTRODUCTION ........................................................................................................ 4 1.1 SECURITY TARGET REFERENCE ...................................................................................................................... 5 1.2 TOE REFERENCE ............................................................................................................................................ 5 1.3 TOE OVERVIEW ............................................................................................................................................. 5 1.4 TOE DESCRIPTION ......................................................................................................................................... 6 1.4.1 TOE Architecture ................................................................................................................................... 7 1.4.2 TOE Documentation .............................................................................................................................. 9 2. CONFORMANCE CLAIMS ........................................................................................................................... -

Tarifas De Servicio/ Deducibles T-Mobile®
Tarifas de servicio/ deducibles T-Mobile® Las tarifas de servicio/deducibles a continuación se aplican a los siguientes programas: JUMP! Plus™ Protección Premium Plus para Dispositivos Nivel 1 Nivel 2 Nivel 3 Tarifa de servicio: $20 por reclamo Tarifa de servicio: $20 por reclamo Tarifa de servicio: $20 por reclamo para daño accidental para daño accidental para daño accidental Deducible: $20 por reclamo Deducible: $50 por reclamo Deducible: $100 por reclamo para pérdida y robo para pérdida y robo para pérdida y robo Cargo por equipo no recuperado: Cargo por equipo no recuperado: Cargo por equipo no recuperado: hasta $200 hasta $300 hasta $500 ALCATEL A30 ALCATEL ONETOUCH Fierce XL LG G Stylo ALCATEL Aspire ALCATEL ONETOUCH POP 7 Samsung Galaxy Tab A 8.0 ALCATEL GO FLIP HTC Desire 530 Samsung Gear S2 ALCATEL LinkZone Hotspot HTC Desire 626s ALCATEL ONETOUCH POP Astro LG G Pad F Coolpad Catalyst LG G Pad X 8.0 Coolpad Rogue LG K10 Kyocera Rally LG K20 LG 450 LG Stylo 3 Plus LG Aristo LG Stylo Plus LG K7 Kyocera Hydro Wave C6740 LG Leon LTE Microsoft Lumia 640 Microsoft Lumia 435 Samsung Galaxy Core Prime T-Mobile LineLink Samsung Galaxy Grand Prime ZTE Avid Plus Samsung Galaxy J3 Prime ZTE Avid Trio Samsung Galaxy J7 ZTE Cymbal Samsung Galaxy Tab E ZTE Falcon Z-917 Hotspot ZTE SyncUp DRIVE ZTE Obsidian ZTE Quartz ZTE Zmax Pro Nivel 4 Nivel 5 Tarifa de servicio: $99 por reclamo Tarifa de servicio: $99 por reclamo para daño accidental para daño accidental Deducible: $150 por reclamo Deducible: $175 por reclamo para pérdida y robo para pérdida -

Barometer of Mobile Internet Connections in Indonesia Publication of March 14Th 2018
Barometer of mobile Internet connections in Indonesia Publication of March 14th 2018 Year 2017 nPerf is a trademark owned by nPerf SAS, 87 rue de Sèze 69006 LYON – France. Contents 1 Methodology ................................................................................................................................. 2 1.1 The panel ............................................................................................................................... 2 1.2 Speed and latency tests ....................................................................................................... 2 1.2.1 Objectives and operation of the speed and latency tests ............................................ 2 1.2.2 nPerf servers .................................................................................................................. 2 1.3 Tests Quality of Service (QoS) .............................................................................................. 2 1.3.1 The browsing test .......................................................................................................... 2 1.3.2 YouTube streaming test ................................................................................................ 3 1.4 Filtering of test results .......................................................................................................... 3 1.4.1 Filtering of devices ........................................................................................................ 3 2 Overall results 2G/3G/4G ............................................................................................................ -

Case 5:18-Cv-06739-VKD Document 27 Filed 07/02/18 Page 1 of 11
Case 5:18-cv-06739-VKD Document 27 Filed 07/02/18 Page 1 of 11 IN THE UNITED STATES DISTRICT COURT FOR THE NORTHERN DISTRICT OF TEXAS DALLAS DIVISION § UNILOC USA, INC. and § UNILOC LUXEMBOURG, S.A., § Civil Action No. 3:18-cv-00560-DCG § Plaintiffs, § § v. § PATENT CASE § LG ELECTRONICS U.S.A., INC., § LG ELECTRONICS MOBILECOMM § U.S.A. INC. and § LG ELECTRONICS, INC., § § Defendant. § JURY TRIAL DEMANDED § FIRST AMENDED COMPLAINT FOR PATENT INFRINGEMENT Plaintiffs, Uniloc USA, Inc. (“Uniloc USA”) and Uniloc Luxembourg, S.A. (“Uniloc Luxembourg”) (together, “Uniloc”), for their First Amended Complaint against defendant, LG Electronics U.S.A. (“LGE”), Inc., LG Electronics Mobilecomm U.S.A., Inc., and LG Electronics, Inc., (“LG Korea”) (together “LG”), allege as follows:1 THE PARTIES 1. Uniloc USA is a Texas corporation having a principal place of business at Legacy Town Center I, Suite 380, 7160 Dallas Parkway, Plano Texas 75024. Uniloc USA also maintains a place of business at 102 N. College, Suite 603, Tyler, Texas 75702. 1 An amended complaint supersedes the original complaint and renders it of no legal effect, King v. Dogan, 31 F.3d 344. 346 (5th Cir. 1994), rendering a motion to dismiss the original complaint moot. See, e.g., Mangum v. United Parcel Services, No. 3:09-cv-0385, 2009 WL 2700217 (N.D. Tex. Aug. 26, 2009). 2988606.v1 Case 5:18-cv-06739-VKD Document 27 Filed 07/02/18 Page 2 of 11 2. Uniloc Luxembourg is a Luxembourg public limited liability company having a principal place of business at 15, Rue Edward Steichen, 4th Floor, L-2540, Luxembourg (R.C.S. -

Terraillon Wellness Coach Supported Devices
Terraillon Wellness Coach Supported Devices Warning This document lists the smartphones compatible with the download of the Wellness Coach app from the App Store (Apple) and Play Store (Android). Some models have been tested by Terraillon to check the compatibility and smooth operation of the Wellness Coach app. However, many models have not been tested. Therefore, Terraillon doesn't ensure the proper functioning of the Wellness Coach application on these models. If your smartphone model does not appear in the list, thank you to send an email to [email protected] giving us the model of your smartphone so that we can activate if the application store allows it. BRAND MODEL NAME MANUFACTURER MODEL NAME OS REQUIRED ACER Liquid Z530 acer_T02 Android 4.3+ ACER Liquid Jade S acer_S56 Android 4.3+ ACER Liquid E700 acer_e39 Android 4.3+ ACER Liquid Z630 acer_t03 Android 4.3+ ACER Liquid Z320 T012 Android 4.3+ ARCHOS 45 Helium 4G a45he Android 4.3+ ARCHOS 50 Helium 4G a50he Android 4.3+ ARCHOS Archos 45b Helium ac45bhe Android 4.3+ ARCHOS ARCHOS 50c Helium ac50che Android 4.3+ APPLE iPhone 4S iOS8+ APPLE iPhone 5 iOS8+ APPLE iPhone 5C iOS8+ APPLE iPhone 5S iOS8+ APPLE iPhone 6 iOS8+ APPLE iPhone 6 Plus iOS8+ APPLE iPhone 6S iOS8+ APPLE iPhone 6S Plus iOS8+ APPLE iPad Mini 1 iOS8+ APPLE iPad Mini 2 iOS8+ 1 / 48 www.terraillon.com Terraillon Wellness Coach Supported Devices BRAND MODEL NAME MANUFACTURER MODEL NAME OS REQUIRED APPLE iPad Mini 3 iOS8+ APPLE iPad Mini 4 iOS8+ APPLE iPad 3 iOS8+ APPLE iPad 4 iOS8+ APPLE iPad Air iOS8+ -

Electronic 3D Models Catalogue (On July 26, 2019)
Electronic 3D models Catalogue (on July 26, 2019) Acer 001 Acer Iconia Tab A510 002 Acer Liquid Z5 003 Acer Liquid S2 Red 004 Acer Liquid S2 Black 005 Acer Iconia Tab A3 White 006 Acer Iconia Tab A1-810 White 007 Acer Iconia W4 008 Acer Liquid E3 Black 009 Acer Liquid E3 Silver 010 Acer Iconia B1-720 Iron Gray 011 Acer Iconia B1-720 Red 012 Acer Iconia B1-720 White 013 Acer Liquid Z3 Rock Black 014 Acer Liquid Z3 Classic White 015 Acer Iconia One 7 B1-730 Black 016 Acer Iconia One 7 B1-730 Red 017 Acer Iconia One 7 B1-730 Yellow 018 Acer Iconia One 7 B1-730 Green 019 Acer Iconia One 7 B1-730 Pink 020 Acer Iconia One 7 B1-730 Orange 021 Acer Iconia One 7 B1-730 Purple 022 Acer Iconia One 7 B1-730 White 023 Acer Iconia One 7 B1-730 Blue 024 Acer Iconia One 7 B1-730 Cyan 025 Acer Aspire Switch 10 026 Acer Iconia Tab A1-810 Red 027 Acer Iconia Tab A1-810 Black 028 Acer Iconia A1-830 White 029 Acer Liquid Z4 White 030 Acer Liquid Z4 Black 031 Acer Liquid Z200 Essential White 032 Acer Liquid Z200 Titanium Black 033 Acer Liquid Z200 Fragrant Pink 034 Acer Liquid Z200 Sky Blue 035 Acer Liquid Z200 Sunshine Yellow 036 Acer Liquid Jade Black 037 Acer Liquid Jade Green 038 Acer Liquid Jade White 039 Acer Liquid Z500 Sandy Silver 040 Acer Liquid Z500 Aquamarine Green 041 Acer Liquid Z500 Titanium Black 042 Acer Iconia Tab 7 (A1-713) 043 Acer Iconia Tab 7 (A1-713HD) 044 Acer Liquid E700 Burgundy Red 045 Acer Liquid E700 Titan Black 046 Acer Iconia Tab 8 047 Acer Liquid X1 Graphite Black 048 Acer Liquid X1 Wine Red 049 Acer Iconia Tab 8 W 050 Acer -

Lg G5 “Friends” Companion Devices Launch in Us
www.LG.com LG G5 “FRIENDS” COMPANION DEVICES LAUNCH IN US Available Now, LG CAM Plus, LG 360 CAM, LG 360 VR, LG TONE via Carriers, Retailers and on LG.COM ENGLEWOOD CLIFFS, N.J., May 24 2016 — Beginning today, LG Electronics’ (LG) customers in the US can get their hands on the highly anticipated LG G5 “Friends” companion devices, including the LG CAM Plus, LG 360 CAM, LG 360 VR and LG TONE Platinum™ on LG.com. The recently launched LG G5 smartphone showcases a new modular design that lets owners easily add LG Friends to transform the G5 into a DSLR-inspired camera, mobile virtual reality viewer and even a 360 video camera. The LG Friends are available now via major US carriers and at retail locations as well as the expanded LG online store – a destination to find great products that help customers get the most out of their favorite LG smartphones. LG.com will also serve as a way to order LG G5 batteries and new charging cradles in the coming weeks – which can be used to charge the G5. In addition, the online store offers batteries and charging cradles for beloved products such as the LG G3, LG G4, and LG V10*. For a limited time only, the store will be rewarding loyal customers with several promotions to celebrate some of LG’s most successful products and innovations. Consumers can take advantage of deep discounts including up to 70 percent off MSRP for, G4 and V10 battery and charger bundles starting for as low as $20.** “The LG ‘Friends’ make the G5 so much more than any other phone on the market. -

LG G6 User Guide
Sprint User Guide A downloadable, printable guide to your LG G6 and its features. Available applications and services are subject to change at any time. Table of Contents GETTING STARTED 1 Introduction 2 About the User Guide 2 Access Instructions on the Phone 2 Launch the Quick Help 2 Access Help 2 Get Support from Sprint Zone 3 Feature Highlights 3 Wide-Angle Cameras 3 FullVision 4 Water and Dust Resistant Properties 5 Set Up Your Phone 7 Parts and Functions 7 Battery Use 9 Charge the Battery 9 Nano SIM Card 10 microSD Card 12 Insert microSD Card 12 Unmount and Remove the microSD Card 13 Format microSD Card 14 Turn Your Phone On and Off 15 Use the Touchscreen 15 Activate Your Phone 16 Complete the Setup Screens 17 Basic Operations 19 Basics 19 Home Screen 19 Phone Settings Menu 20 Portrait and Landscape Screen Orientation 20 Capture Screenshots 21 Applications 21 Launch Applications 21 Preinstalled Apps 22 i Fingerprint Recognition 23 Precautions for Fingerprint Recognition 23 Registering Fingerprints 24 Managing Fingerprints 25 Unlocking the Screen with a Fingerprint 25 Multi-tasking Feature 25 Multi-Window 25 Overview Screen 26 Phone Number 26 Airplane Mode 27 Enter Text 27 QWERTY Keyboard 28 Phone Keyboard 29 Tips for Editing Text 30 Google Account 32 Create a Google Account 32 Create an Exchange Account 33 Sign into Your Google Account 34 Factory Reset Protection 34 Google Play Store 35 Find and Install an App 35 Request a Refund for a Paid App 36 Update an App 36 Uninstall an App 37 Get Help with Google Play 37 Lock and Unlock Your -

2016 LG G4 and V10 February Promo Issue Date
2016 LG G4 and V10 February Promo Issue Date: 2/1/2016 Program Name: February 2016 G4 and V10 Promo Program Period: 2/12/2016 through 3/11/2016 Program Offer: Consumers age 18 or older purchasing an eligible LG G4 or LG V10 at eligible Verizon Wireless Locations and participating Verizon Authorized Dealers or www.verizonwireless.com on device payment plan between February 12th 2016 and March 11th, 2016 (the “Program Period”) will be qualified to receive an LG Visa® Prepaid Card by mail-in rebate. Consumers may submit their claim to LG Electronics USA by way of our online portal The eligible LG G4 and LG V10 products for the Promotion Redemption are as follows: ELIGIBLE SMARTPHONE MODEL COLOR LG G4 VS986LD GENUINE LEATHER BLACK LG G4 VS986VK METALLIC GRAY LG G4 VS986W CERAMIC WHITE LGV10 VS990 SPACE BLACK LGV10 VS990W LUXE WHITE 2016 LG G4 and V10 February Promotion Offer Amounts: Consumers purchasing eligible LG G4 and LG V10 models are eligible to receive the corresponding prepaid card value below: LG G4 OR V10 PREPAID CARD ELIGIBLEMODEL PREPAIDCARD VALUE LG G4 VS986 $100 LG V10 VS990 $100 Program Requirements: Consumer Redemption Process Online only: Consumer can enter their claim at www.acbincentives.com/LGMUFebruary and upload (1) the original, dated sales receipt or a legible copy of the original dated sales receipt from a participating retailer dated between February 12th 2016 & march 11th 2016 (2) the original UPC & IMEI codes for the device taken from the packaging (or a legible copy of the codes). Consumer Redemption Rules: End-user consumer purchasers age 18 or older who purchase qualifying new LG G4 or LG V10 models on device payment plan at eligible Verizon Wireless Locations and participating Verizon Authorized Dealers or www.verizonwireless.com as a resident in the 50U.S. -

Meet Your Phone
Meet your About your phone Setting up your phone Your phone already has a SIM card installed. Charge your phone phone. Before turning on your phone, charge it Optional: Insert the microSD card fully. Back cameras Earpiece (wide-angle camera 1. With the phone’s screen facing up, insert and standard camera) Your phone comes with an fast charging Headset jack the tool that came with your phone into the hole on the SIM/microSD card tray to charger and a USB Type-C cable. Plug one partially eject the tray. Then, carefully pull end of the USB Type-C cable to the USB/ Front charger port (as shown below) and the camera the card tray out. 2. Insert the microSD card with the gold other end into the charger. Then plug the Flash contacts facing down and toward the charger into an outlet. Volume SIM/ Power/ buttons microSD Lock button, phone (as shown below). card slot Fingerprint 3. Carefully insert the card tray back into the sensor slot and close it. Wireless charging spot NOTE: The microSD card is sold separately. USB/Charger port Speaker Microphone SIM Card microSD card Para la versión en español, visite verizonwireless.com/Support WARNING: Only use approved chargers with your NOTE: Devices and software are constantly device. Incompatible chargers or tampering with evolving—the screen images and icons you see the charger port could damage your device and here are for reference only. void the warranty. Using your phone Learning more Managing your account Additional information Your wireless device and third-party services Turning your phone on/off Home screen mode The Help app gives you tips and My Verizon Mobile app Verizon Wireless is the mobile carrier associated To turn your phone on, press and hold the You can select one of 3 modes: other information on how to use Manage your account, track your with this wireless device, but many services and features offered through this device are provided Power/Lock button. -
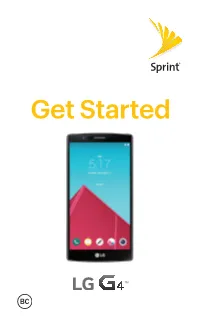
Get Started Welcome! Thank You for Choosing Sprint
Get Started Welcome! Thank you for choosing Sprint. This guide helps you get started with your HD Voice capable* LG G4™. ReadyNow – For more help in getting up and running with your new phone, take advantage of exclusive Ready Now resources at sprint.com/readynow. Visit sprint.com/support for ongoing support and resources. There, you’ll find user guides, tutorials, and more to help you get the most out of your phone. Note: Available applications and services are subject to change at any time. * HD Voice capability is available in select markets. Visit sprint.com/coverage for more information. Your LG G4 Notification Earpiece LED Front-Facing Camera Lens Proximity Sensor Touchscreen Back Key Recent Home Key Apps Key Back-Facing IR (Infrared) Camera Lens LED Flash Power/ Laser Detection Lock Button Auto-focus Volume Buttons Speaker 3.5mm Headset Jack Charger/ Accessory Port Microphone Activate Your Phone Charge the Battery Your phone’s battery should have enough charge to get started, but it’s a good idea to charge it fully before you get going. Insert the USB connector into the charger/accessory port. Plug the other end of the USB cable into the AC adapter and plug the AC adapter into an electrical outlet. Before turning on your phone: If you purchased your phone at a Sprint Store, it is probably activated and ready to use. If you received your phone in the mail, and it is for a new Sprint account or a new phone number, your phone is designed to activate automatically when you first turn it on. -

LG G6 USER GUIDE Copyright©2017 LG Electronics, Inc
LG G6 USER GUIDE Copyright©2017 LG Electronics, Inc. All rights reserved. LG and the LG logo are registered trademarks of LG Corp. G6 is a trademark of LG Electronics, Inc. All other trademarks are the property of their respective owners. Important Customer Information 1 Before you begin using your new phone Included in the box with your phone are separate information leaflets. These leaflets provide you with important information regarding your new device. Please read all of the information provided. This information will help you to get the most out of your phone, reduce the risk of injury, avoid damage to your device, and make you aware of legal regulations regarding the use of this device. It’s important to review the Product Safety and Warranty Information guide before you begin using your new phone. Please follow all of the product safety and operating instructions and retain them for future reference. Observe all warnings to reduce the risk of injury, damage, and legal liabilities. 2 Table of Contents Important Customer Information...............................................1 Table of Contents .......................................................................2 The Basics ...................................................................................5 Phone Overview .................................................................................................... 5 Help ....................................................................................................................... 8 Setting Up Your Phone .......................................................................................