A Baby with Bruises / Holland and Ciener • Vol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Skin Injuries – Can We Determine Timing and Mechanism?
Skin injuries – can we determine timing and mechanism? Jo Tully VFPMS Seminar 2016 What skin injuries do we need to consider? • Bruising • Commonest accidental and inflicted skin injury • Basic principles that can be applied when formulating opinion • Abrasions • Lacerations }we need to be able to tell the difference • Incisions • Stabs/chops • Bite marks – animal v human / inflicted v ‘accidental’ v self-inflicted Our role…. We are often/usually/always asked…………….. • “What type of injury is it?” • “When did this injury occur?” • “How did this injury occur?” • “Was this injury inflicted or accidental?” • IS THIS CHILD ABUSE? • To be able to answer these questions (if we can) we need knowledge of • Anatomy/physiology/healing - injury interpretation • Forces • Mechanisms in relation to development, plausibility • Current evidence Bruising – can we really tell which bruises are caused by abuse? Definitions – bruising • BLUNT FORCE TRAUMA • Bruise =bleeding beneath intact skin due to BFT • Contusion = bruise in deeper tissues • Haematoma - extravasated blood filling a cavity (or potential space). Usually associated with swelling • Petechiae =Pinpoint sized (0.1-2mm) hemorrhages into the skin due to acute rise in venous pressure • medical causes • direct forces • indirect forces Medical Direct Indirect causes mechanical mechanical forces forces Factors affecting development and appearance of a bruise • Properties of impacting object or surface • Force of impact • Duration of impact • Site - properties of body region impacted (blood supply, -

Protein C Deficiency
Protein C deficiency Description Protein C deficiency is a disorder that increases the risk of developing abnormal blood clots; the condition can be mild or severe. Individuals with mild protein C deficiency are at risk of a type of blood clot known as a deep vein thrombosis (DVT). These clots occur in the deep veins of the arms or legs, away from the surface of the skin. A DVT can travel through the bloodstream and lodge in the lungs, causing a life-threatening blockage of blood flow known as a pulmonary embolism (PE). While most people with mild protein C deficiency never develop abnormal blood clots, certain factors can add to the risk of their development. These factors include increased age, surgery, inactivity, or pregnancy. Having another inherited disorder of blood clotting in addition to protein C deficiency can also influence the risk of abnormal blood clotting. In severe cases of protein C deficiency, infants develop a life-threatening blood clotting disorder called purpura fulminans soon after birth. Purpura fulminans is characterized by the formation of blood clots in the small blood vessels throughout the body. These blood clots block normal blood flow and can lead to localized death of body tissue ( necrosis). Widespread blood clotting uses up all available blood clotting proteins. As a result, abnormal bleeding occurs in various parts of the body, which can cause large, purple patches on the skin. Individuals who survive the newborn period may experience recurrent episodes of purpura fulminans. Frequency Mild protein C deficiency affects approximately 1 in 500 individuals. Severe protein C deficiency is rare and occurs in an estimated 1 in 4 million newborns. -

I, Paul Knoebl
Knoebl 2020-12-09 Public Declaration of Interests and Confidentiality Undertaking of European Medicines Agency (EMA), Scientific Committee members and experts Public declaration of interests I, Paul Knoebl Organisation/Company: Medical University of Vienna Country: Austria do hereby declare on my honour that, to the best of my knowledge, the only direct or indirect interests I have in the pharmaceutical industry are those listed below: 2.1 Employment No interest declared 2.2 Consultancy Period Company Products Therapeutic Indication 01/2009-(current) Novo Nordisk acquired hemophilia, congenital hemophilia, rare bleeding disorders 01/2012-02/2019 Baxalta (now Shire) thrombotic thrombocytopenic purpura purpura fulminans acquired hemophilia 01/2012-01/2016 Alexion thrombotic microangiopathy 03/2010-07/2018 Ablynx thrombotic microangiopathy 07/2018-(current) Sanofi Genzyme thrombotic microangiopathy 02/2019-(current) Takeda thrombotic microangiopathy Acquired hemophilia 2.3 Strategic advisory role No interest declared 2.4 Financial interests Classified as public by the European Medicines Agency DOI Form Version-number: 4 Knoebl 2020-12-09 2 No interest declared 2.5 Principal investigator Period Company Products Therapeutic Indication 01/2013-(current) Baxalta, then Shire, now Takeda BAX930 Upshaw Schulman Syndrome 01/2013-(current) Novo Nordisc Concizumab Hemophilia 09/2010-04/2014 Gilead Ambisome fungal infections 09/2010-04/2014 MSD Posaconazol fungal infections 03/2010-(current) Ablynx caplacizumab thrombotic thrombocytopenic purpura 04/2010-01/2012 -
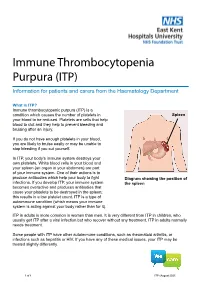
Immune Thrombocytopenia Purpura (ITP)
Immune Thrombocytopenia Purpura (ITP) Information for patients and carers from the Haematology Department What is ITP? Immune thrombocytopenic purpura (ITP) is a condition which causes the number of platelets in Spleen your blood to be reduced. Platelets are cells that help blood to clot and they help to prevent bleeding and bruising after an injury. If you do not have enough platelets in your blood, you are likely to bruise easily or may be unable to stop bleeding if you cut yourself. In ITP, your body’s immune system destroys your own platelets. White blood cells in your blood and your spleen (an organ in your abdomen) are part of your immune system. One of their actions is to produce antibodies which help your body to fight Diagram showing the position of infections. If you develop ITP, your immune system the spleen becomes overactive and produces antibodies that cause your platelets to be destroyed in the spleen; this results in a low platelet count. ITP is a type of autoimmune condition (which means your immune system is acting against your body rather than for it). ITP in adults is more common in women than men. It is very different from ITP in children, who usually get ITP after a viral infection but who recover without any treatment. ITP in adults normally needs treatment. Some people with ITP have other autoimmune conditions, such as rheumatoid arthritis, or infections such as hepatitis or HIV. If you have any of these medical issues, your ITP may be treated slightly differently. 1 of 8 ITP (August 2021) A normal platelet count is between 150 and 400 thousand million platelets per litre of blood. -

Bleeds and Bruises in Children with Haemophilia
Bleeds and Bruises in CHildren WiTH HaeMOPHilia MusCle ANd/or JoiNt Bleeds Call the parent/guardian P.r.i.C.e. siGNs oF A serious HeAd Bleed P : Protection * Headache. Lower Limb: Take weight off the joint or muscle * drowsiness. Upper Limb: No carrying using affected arm * Nausea. r : rest * Vomiting. • Rest means rest! * unsteady Balance. • Try not to allow use of the joint or muscle where * irritability. possible. * Confusion. * seizures. i : ice * loss of consciousness. • Regular ice packs can help with pain & reduce swelling. • Put an ice pack over the affected area for 20 minutes. Repeat every two hours. DO NOT leave the ice pack on for more than 20 minutes siGNs oF A soFt tissue DO NOT place ice pack directly on skin (Use a tea Bleed towel/cold pack cover) * Bruising, discolouring of skin. C : Compression * Mild swelling. • Use an elasticated bandage to compress the affected area to reduce swelling. e : elevation • Elevate the affected limb to help reduce swelling. siGNs oF AN ABdoMiNAl • Keep the affected joint or muscle above the level of the Bleed heart. * Bloody, black or tar-like First Aid bowel motions. * red or brown urine. Mouth & Gum Bleeds * Pain. These can be hard to control because clots that form are * Vomiting of blood (blood washed away by saliva or knocked off by the tongue or food. Try giving the child an ice cube or ice pop to suck. may be red or black). These bleeds may need treatment by parents or the treatment centre. Nosebleeds siGNs oF BleediNG iNto tHe Tilt head forward and pinch the bridge of the nose below the bone for 10 - 20 minutes and / or put an ice-pack on JoiNts or MusCles the bridge of the nose for not more than 5 minutes. -

Isolated Plantar Vein Thrombosis Resembling a Corn with a Bruise
JE Hahm, et al pISSN 1013-9087ㆍeISSN 2005-3894 Ann Dermatol Vol. 31, No. 1, 2019 https://doi.org/10.5021/ad.2019.31.1.66 CASE REPORT Isolated Plantar Vein Thrombosis Resembling a Corn with a Bruise Ji Eun Hahm, Kang Su Kim, Jae Won Ha, Chul Woo Kim, Sang Seok Kim Department of Dermatology, Kangdong Sacred Heart Hospital, College of Medicine, Hallym University, Seoul, Korea Plantar vein thrombosis, rarely-reported disease, is usually or callus, plantar fibromatosis, or plantar verruca1. Among accompanied by pain and tenderness in the plantar region laborers, they may develop from excess pressure on the and should be differentiated from other dermatological con- bony prominences of the feet, repetitive uneven friction ditions causing plantar pain, such as hemorrhagic corn/cal- from footwear, or gait abnormalities. Plantar vein throm- lus, plantar epidermal cyst, verruca, or plantar fibromatosis. bosis is a rare condition causing plantar pain. The exact A 52-year-old man presented with a violaceous tender sub- cause of plantar vein thrombosis is yet unclear, but predis- cutaneous nodule overlying a hyperkeratotic plaque on his posing conditions, such as prior trauma, surgery, paraneo- sole. Initially, he thought it was a corn and applied keratolytic plastic syndromes, or coagulation disorders have been agents, which failed to work. Sonography revealed a well-de- described. To date, there is no established treatment ex- marcated mass with increased peripheral vascularity. His cept surgical excision, but reportedly, nonsteroidal anti-in- pain was relieved after a complete wide excision, which con- flammatory drug or heparin with elastic bandage is known firmed the mass to be plantar vein thrombosis after histo- to be effective for symptomatic control2-5. -

Bruise, Contusion & Ecchymosis Conventions
Bruise, Contusion and Ecchymosis MedDRA Proactivity Proposal Implementation MedDRA Version 16.0 I. MSSO Recognized Definitions of Concepts and Terms The MSSO has designated Dorland’s Illustrated Medical Dictionary as the standard reference for medical definitions. The following definitions are cited from Dorland’s 27th edition: Bruise – A superficial injury produced by impact without laceration; a contusion Contusion – A bruise; an injury of a part without a break in the skin Ecchymosis – A small hemorrhagic spot, larger than a petechia, in the skin or mucous membrane forming a nonelevated, rounded or irregular, blue or purplish patch. Hematoma – A localized collection of blood, usually clotted, in an organ, space, or tissue, due to a break in the wall of a blood vessel. Hemorrhage – The escape of blood from the vessels; bleeding. Petechia – A pinpoint, non-raised, perfectly round, purplish red spot caused by intradermal or submucous hemorrhage. Additional comments regarding the definitions: Bruise and contusion are synonymous, and are often used in a colloquial context. Bruise and contusion are each considered a result of injury. Bruise and contusion have been used to describe minor hemorrhage within tissue, where traumatized blood vessels leak blood into the interstitial space. Commonly, capillaries and sometimes venules are injured within skin, subcutaneous tissue, muscle, or bone. In addition to trauma, the terms bruise, ecchymosis, and to a lesser extent, contusion, have also been used as clinical signs of disorders of platelet function, coagulopathies, venous congestion, allergic reactions, etc. Hemorrhage may be used to describe blood escaping from vessels and retained in the interstitial space, and perhaps more commonly, to describe the escape of blood from vessels, and flowing freely external to the tissues. -

Understanding Haemophilia
Understanding haemophilia Understanding haemophilia Contents Introduction 3 Haemophilia and your child 4 What is haemophilia? 5 What causes haemophilia? 5 Can females have haemophilia? 6 Carriers 8 Who is affected by haemophilia? 9 How severe is haemophilia? 9 Signs and symptoms of haemophilia 11 How is haemophilia diagnosed? 14 Diagnosis 14 Treatment 16 Port-a-cath 19 Managing joint bleeds with PRICE 19 Gene therapy 21 Possible complications of haemophilia 22 Inhibitors 22 Joint damage 22 Medical and dental treatment 23 Surgery Circumcision Dental care Medicines Vaccinations Bleeding disorder card Living with haemophilia 26 Sport and exercise 27 School, college and work 28 Travel 29 Pregnancy and haemophilia 30 Glossary of terms 32 About The Haemophilia Society 33 2 Understanding haemophilia Introduction This booklet is about haemophilia A and B. It gives a general overview of haemophilia and information on diagnosing, treating and living with the condition that we hope will answer your main questions. It has been written for people directly affected by haemophilia and for anyone interested in learning about haemophilia. If you are a parent and your child has recently been diagnosed with haemophilia you may be feeling quite overwhelmed. Remember, you’re not alone and many families are facing the same concerns and issues. Please do get in touch – we have lots of support and information available as well as services for parents and children. You can find out more via our website or Facebook pages, by emailing [email protected] or calling us on 020 7939 0780. The outlook is now the best it has ever been for people with haemophilia in the UK. -

Immune Thrombocytopenic Purpura in a Twin Girl Revealed by a Traumatic Injury in Parakou (North Benin)
Immune thrombocytopenic purpura in a twin girl revealed by a traumatic injury in parakou (North Benin) Adedemy JD 1*, Noudamadjo A 1, Kpanidja G 1, Agossou J 1, Agbeille Mohamed F 1, Dovonou CA 2 1 Mother and Child Department, Parakou Teaching Hospital, Republic of Benin, and Faculty of Medicine, University of Parakou West Africa 2 Department of Medicine, Parakou Teaching Hospital, and Faculty of Medicine, University of Parakou, West Africa Abstract Background: ITP seems to be rare but in tropical settings thrombocytopenia is often encountered among children. Objective: Authors through this case report are putting emphasy on the diagnosis and management of ITP in a 4 year old twin girl admitted in the pediatric emergency ward for hematuria and bleeding from various origins seen in the context of a domestic trauma. Results: The various clinical signs have been analyzed to confirm ITP through exclusion of other possible health conditions. The management of ITP depend on the severity of clinical signs and in some cases the situations can be life threatening. In this case report, Blood transfusion and corticosteroids were the main treatment tools. The hospital stay was about 47 days and an ambulatory follow up was conducted for almost 6 months. Conclusion: In the context of various bleeding disorders, hematuria and thrombocytopenia, autoimmune thrombocytopenia in a twin girl was revealed by a domestic trauma. Citation: Adedemy JD, Noudamadjo A, Kpanidja G, Agossou J, Agbeille MF, Dovonou CA (2019) Immune Thrombocytopenic Purpura in a twin girl revealed by a traumatic injury in Parakou (North Benin). Adv Pediatr Res 6:27. -

How Significant Is Bleeding in Antiphospholipid Antibody
Treatment of Anti-Phospholipid Syndrome and Prothrombin Deficiency with Plasma Exchange Lowell Tilzer KU Medical Center Department of Pathology & Lab Medicine Case 72 YEARS OLD FEMALE PRESENTED TO KUMC WITH BLEEDING FOLLOWING ROUTINE HEMORRHOIDECTOMY SURGERY Past Medical History • Surgeries: Bilateral tubal ligation, appendectomy, partial hysterectomy, bilateral bunion surgery – No bleeding complications • 2008: Presented to ER with chest pain – Incidentally found prolonged PT and PTT + Lupus anticoagulant and anticardiolipin antibodies • 2009: Melanotic stool with severe anemia (Hb 4) – Blood transfusion (>10 units) – Attributed to long-term use of Aspirin Brief course Initial Work-up Reported Normal Test Value Range PT/INR 2.2 (HIGH) 0.8-1.2 PTT 87.4 (HIGH) 24.0-40.0 PT mixing study @ 60 mins 1.5 (HIGH) PTT mixing study @ 60 mins 82.8 (HIGH) Factor 2 assay 10% (LOW) 50-150% Factor 5 assay 78% 50-150% Factor 7 assay 153% (HIGH) 50-150% Factor 8 assay 235% (HIGH) 50-150% Factor 10 assay 68% 50-150% 300 Linear dilutions 250 200 150 100 % Normal control 50 0 Factor 2 Factor 5 Factor 7 Factor 8 Factor 10 Additional coagulation study Test Result Interpretation dRVVT Prolonged Lupus anticoagulant Abs Hexagonal Lupus Positive Lupus anticoagulant Abs anticoagulant Anti-b2 GPI IgG Positive Anti-b2 GPI IgM Positive Support APS diagnosis Anti-cardiolipin IgG Positive Anti-cardiolipin IgM Positive There is NO Factor 2 inhibitor, therefore previous result of low Factor 2 inhibitor <0.4 BU Factor 2 level was due to lupus (activity-based) anticoagulant Abs against phospholipids in the assay. Algorithm for Screening tests (PT/INR, aPTT) coagulopathies Mixing study Heparin, DTI PL dependent assays (aPTT or dRVVT + Hexa) PTT PT PT PTT LA+ Factor LA- Intrinsic Common Extrinsic inhibitors pathway pathway pathway Revised Criteria for Antiphospholipid Syndrome (APS) (Sydney Criteria) APS: ≥ 1 Laboratory Criteria AND ≥ 1 Clinical Criteria • Laboratory Criteria: “≥ 2 occasions 3 months apart” 1. -

Cutaneous Findings in Patients on Anticoagulants Caleb Creswell, MD Dermatology Specialists Disclosure Information
Cutaneous findings in patients on Anticoagulants Caleb Creswell, MD Dermatology Specialists Disclosure Information • I have no financial relationships to disclose Objectives 1) Identify underlying causes of actinic or senile purpura 2) Recognize coumadin skin necrosis and understand proper treatment 3) Recognize heparin skin necrosis and understand that underlying HIT is often present Actinic (Senile) Purpura • Common on forearms of elderly individuals • Most important factor is chronic sun exposure – Thins dermal collagen and blood vessel walls • Anticoagulants may exacerbate but are rarely the main culprit Actinic Purpura Actinic Purpura Leukocytoclastic Vasculitis • Don’t mistake LCV for actinic purpura Ecchymoses • No topical agents have been shown to speed resorption of RBCs and hemosiderin • Pulsed-dye Laser can help Coumadin Induced Skin Necrosis • Coumadin: Occurs between 3-5 days after initiating therapy – Due to transient protein C deficiency – Increased risk with intrinsic protein C deficiency – Occurs in areas with significant adipose tissue – Treatment: Heparinize and continue coumadin Coumadin Induced Skin Necrosis Other Coumadin Skin Reactions • Extremely rare cause of morbilliform drug rash • Can cause leukocytoclastic vasculitis – Can occur weeks to months after starting medication Photo of Morbilliform CADR Leukocytoclastic Vasculitis Heparin Induced Skin Necrosis • Heparin: Occurs 1-14 days after starting – Often starts at injection site and spreads – Due to HIT Type II (Thrombocytopenia will be present) Heparin Induced -

Extensive Purpura and Necrosis of the Leg
PHOTO CHALLENGE Extensive Purpura and Necrosis of the Leg Michael Musharbash, MD; Lida Zheng, MD; Lauren Guggina, MD A 57-year-old woman presented with expanding purpura on the left leg of 2 weeks’ duration following a recent hema- topoietic stem cell transplant for refractory diffuse large B-cell lymphoma. Prior to dermatologic consultation, the patient had been hospitalizedcopy for 2 months following the transplant due to Clostridium difficile colitis, Enterococcus faecium bactere- mia, cardiac arrest, delayed engraftment with pancytopenia, and atypical hemolytic uremic syndrome with acute renal failure requiring hemodialysis and treatment with eculizumab. Hernot care team in the hospital initially noticed a small purpuric lesion on the posterior aspect of the left knee. The patient subsequently developed persistent fevers and expansion of the lesion, which prompted consultation of the dermatology ser- vice. Physical examination revealed a 22×10-cm, rectangular, indurated, purpuric plaque with central dusky, violaceous to black necrosis with superficial skin sloughing and peripheral dusky erythema extending from the inner thigh to the lower leg. The left distal leg felt cool, and both dorsalis pedis and posterior tibial pulses were absent. Laboratory test results revealed neutropenia and thrombocytopenia 3 3 Do 3 3 (white blood cell count, 0.2×10 /mm [reference range, 5–10×10 /mm ]; hematocrit, 23.2% [reference range, 41%–50%]; platelet count, 105×103/µL [reference range, 150–350×103/µL]). A punch biopsy was performed. WHAT’S THE DIAGNOSIS? a. disseminated aspergillosis b. disseminated intravascular coagulation c. disseminated mucormycosis d. purpura fulminans e. pyodermaCUTIS gangrenosum PLEASE TURN TO PAGE E2 FOR THE DIAGNOSIS From the Department of Dermatology, Northwestern Memorial Hospital, Chicago, Illinois.