Efavirenz) Capsules and Tablets 3 Rx Only
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1: Clinical Pharmacokinetics 1
1: CLINICAL PHARMACOKINETICS 1 General overview: clinical pharmacokinetics, 2 Pharmacokinetics, 4 Drug clearance (CL), 6 Volume of distribution (Vd), 8 The half-life (t½), 10 Oral availability (F), 12 Protein binding (PB), 14 pH and pharmacokinetics, 16 1 Clinical pharmacokinetics General overview General overview: clinical pharmacokinetics 1 The ultimate aim of drug therapy is to achieve effi cacy without toxicity. This involves achieving a plasma concentration (Cp) within the ‘therapeutic window’, i.e. above the min- imal effective concentration (MEC), but below the minimal toxic concentration (MTC). Clinical pharmacokinetics is about all the factors that determine variability in the Cp and its time-course. The various factors are dealt with in subsequent chapters. Ideal therapeutics: effi cacy without toxicity Minimum Toxic Concentration (MTC) Ideal dosing Minimum Effective Concentration (MEC) Drug concentration Time The graph shows a continuous IV infusion at steady state, where the dose-rate is exactly appropriate for the patient’s clearance (CL). Inappropriate dosing Dosing too high in relation to the patient’s CL – toxicity likely Minimum Toxic Concentration (MTC) Minimum Effective Concentration (MEC) Dosing too low in relation to the Drug concentration patient’s CL – drug may be ineffective Time Some reasons for variation in CL Low CL High CL Normal variation Normal variation Renal impairment Increased renal blood fl ow Genetic poor metabolism Genetic hypermetabolism Liver impairment Enzyme induction Enzyme inhibition Old age/neonate 2 General overview Clinical Pharmacokinetics Pharmacokinetic factors determining ideal therapeutics If immediate effect is needed, a loading dose (LD) must be given to achieve a desired 1 concentration. The LD is determined by the volume of distribution (Vd). -

<I>Efavirenz</I>, New Therapeutic Agents for AIDS
CONFERENCE REPORTS 295 CHIMIA 1999. 53. NO.6 CONFERENCE REPORTS Chimia 53 (1999) 295-304 © Neue Schweizerische Chemische Gesellschaft ISSN 0009-4293 Second Swiss/German Meeting on Medicinal Chemistry Fruhjahrsversammlung 1999 der Neuen Schweizerischen Chemischen Gesellschaft (NSCG) 22./23. Marz 1999, Basel Vier Mini-Symposia uber Virologie, Multidrug Resistance, Immunologie und Gene Therapie Organisiert von der Sektion Medizinische Ghemie der NSGG, der Fachgruppe fUr Medizinische Chemie der GDGh und der Basler Chemischen Gesellschatt mit Unterstutzung der Pharmazeutischen Industrie. Report by the Research Team of G. Folkers' 'Correspondence: Prof. Dr. G. Folkers Department of Pharmacy Winterthurerstrasse 190 CH-8057Zi.irich E-Mail: [email protected] Discovery of Indinavir and Efavirenz, New Therapeutic Agents for AIDS Terry A. Lyle, Merck Research Laboratories, West Point, PA, USA For the treatment of HIV infection, two enzymes are of major interest: The HIV-l Protease (PR) and the Reverse- Tran- scriptase (RT). The HIV -Protease, an aspartic-acid pro- tease active as a dimer, is responsible for H 0 the cleavage of polypeptides assembled at o N~' the cell membrane. The inhibition of the Y I( ; N 1\ 0 = H protease-mediated cleavage of the viral "0 o o o precursor polyproteins results in the pro- duction of noninfectious progeny viral par- ticles. The development of lndinavir start- ed with screening a collection of renin inhibitors. A seven-amino-acid analog 1 1 which contains the hydroxyethylene tran- CONFERENCE REPORTS 296 CHIMIA 1999, 53. No.6 prevents the spread of the virus. Different nucleoside inhibitors like AZT, ddI, ddC, d4T and 3TC are already known, but new QH non-nucleoside inhibitors (NNRTI) are U'O developed to decrease the cytotoxicity and N : to improve the selectivity of the viral polymerases vs. -

2D6 Substrates 2D6 Inhibitors 2D6 Inducers
Physician Guidelines: Drugs Metabolized by Cytochrome P450’s 1 2D6 Substrates Acetaminophen Captopril Dextroamphetamine Fluphenazine Methoxyphenamine Paroxetine Tacrine Ajmaline Carteolol Dextromethorphan Fluvoxamine Metoclopramide Perhexiline Tamoxifen Alprenolol Carvedilol Diazinon Galantamine Metoprolol Perphenazine Tamsulosin Amiflamine Cevimeline Dihydrocodeine Guanoxan Mexiletine Phenacetin Thioridazine Amitriptyline Chloropromazine Diltiazem Haloperidol Mianserin Phenformin Timolol Amphetamine Chlorpheniramine Diprafenone Hydrocodone Minaprine Procainamide Tolterodine Amprenavir Chlorpyrifos Dolasetron Ibogaine Mirtazapine Promethazine Tradodone Aprindine Cinnarizine Donepezil Iloperidone Nefazodone Propafenone Tramadol Aripiprazole Citalopram Doxepin Imipramine Nifedipine Propranolol Trimipramine Atomoxetine Clomipramine Encainide Indoramin Nisoldipine Quanoxan Tropisetron Benztropine Clozapine Ethylmorphine Lidocaine Norcodeine Quetiapine Venlafaxine Bisoprolol Codeine Ezlopitant Loratidine Nortriptyline Ranitidine Verapamil Brofaramine Debrisoquine Flecainide Maprotline olanzapine Remoxipride Zotepine Bufuralol Delavirdine Flunarizine Mequitazine Ondansetron Risperidone Zuclopenthixol Bunitrolol Desipramine Fluoxetine Methadone Oxycodone Sertraline Butylamphetamine Dexfenfluramine Fluperlapine Methamphetamine Parathion Sparteine 2D6 Inhibitors Ajmaline Chlorpromazine Diphenhydramine Indinavir Mibefradil Pimozide Terfenadine Amiodarone Cimetidine Doxorubicin Lasoprazole Moclobemide Quinidine Thioridazine Amitriptyline Cisapride -

Sustiva, INN-Efavirenz
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT SUSTIVA 50 mg hard capsules SUSTIVA 100 mg hard capsules SUSTIVA 200 mg hard capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION SUSTIVA 50 mg hard capsules Each hard capsule contains 50 mg of efavirenz. Excipient with known effect Each hard capsule contains 28.5 mg of lactose (as monohydrate). SUSTIVA 100 mg hard capsules Each hard capsule contains 100 mg of efavirenz. Excipient with known effect Each hard capsule contains 57.0 mg of lactose (as monohydrate). SUSTIVA 200 mg hard capsules Each hard capsule contains 200 mg of efavirenz. Excipient with known effect Each hard capsule contains 114.0 mg of lactose (as monohydrate). For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Hard capsule SUSTIVA 50 mg hard capsules Dark yellow and white, printed with "SUSTIVA" on the dark yellow cap and "50 mg" on the white body. SUSTIVA 100 mg hard capsules White, printed with "SUSTIVA" on the body and "100 mg" on the cap. SUSTIVA 200 mg hard capsules Dark yellow, printed with "SUSTIVA" on the body and "200 mg" on the cap. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications SUSTIVA is indicated in antiviral combination treatment of human immunodeficiency virus-1 (HIV- 1) infected adults, adolescents and children 3 months of age and older and weighing at least 3.5 kg. SUSTIVA has not been adequately studied in patients with advanced HIV disease, namely in patients with CD4 counts < 50 cells/mm3, or after failure of protease inhibitor (PI) containing regimens. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

CPIC Guideline for Pharmacogenetics-Guided Warfarin Dosing – Supplement V2.0 1 Table of Contents Guideline Updates
Supplement to: Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for Pharmacogenetics-guided Warfarin Dosing: 2016 Update Julie A. Johnson1, Kelly Caudle2, Li Gong3, Michelle Whirl-Carrillo3, C. Michael Stein4, Stuart A. Scott5, Ming Ta Michael Lee6 , Brian F. Gage7, Stephen E. Kimmel8,9, Minoli A. Perera10, Jeffrey L. Anderson11, Munir Pirmohamed12, Teri E. Klein3, Nita A. Limdi13, Larisa H. Cavallari1, Mia Wadelius14 1Department of Pharmacotherapy and Translational Research, College of Pharmacy, and Center for Pharmacogenomics, University of Florida, Gainesville, Florida, USA 2Department of Pharmaceutical Sciences, St. Jude Children’s Research Hospital, Memphis, TN 3Department of Genetics, Stanford University, Stanford, California, USA 4Division of Clinical Pharmacology Vanderbilt Medical School, Nashville, TN, USA 5Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY, USA 6Laboratory for International Alliance on Genomic Research, RIKEN Center for Integrative Medical Sciences, Yokohama, Japan; National Center for Genome Medicine; Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan; Genomic Medicine Institute Geisinger Health system, Danville, PA 7Department of Internal Medicine, Washington University in St. Louis, St. Louis, Missouri 8Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA 9Department of Medicine and Department of Biostatistics and Epidemiology, University of Pennsylvania -

SUSTIVA Safely and Effectively
HIGHLIGHTS OF PRESCRIBING INFORMATION • Embryo-Fetal Toxicity: Avoid administration in the first trimester of These highlights do not include all the information needed to use pregnancy as fetal harm may occur. (5.6, 8.1) SUSTIVA safely and effectively. See full prescribing information for • Hepatotoxicity: Monitor liver function tests before and during treatment in SUSTIVA. patients with underlying hepatic disease, including hepatitis B or C SUSTIVA (efavirenz) capsules for oral use coinfection, marked transaminase elevations, or who are taking medications associated with liver toxicity. Among reported cases of SUSTIVA (efavirenz) tablets for oral use hepatic failure, a few occurred in patients with no pre-existing hepatic Initial U.S. Approval: 1998 disease. (5.8, 6.1, 8.6) ---------------------------INDICATIONS AND USAGE--------------------------- • Rash: Rash usually begins within 1-2 weeks after initiating therapy and SUSTIVA is a non-nucleoside reverse transcriptase inhibitor indicated in resolves within 4 weeks. Discontinue if severe rash develops. (5.7, 6.1, 17) combination with other antiretroviral agents for the treatment of human • Convulsions: Use caution in patients with a history of seizures. (5.9) immunodeficiency virus type 1 infection in adults and in pediatric patients at • Lipids: Total cholesterol and triglyceride elevations. Monitor before least 3 months old and weighing at least 3.5 kg. (1) therapy and periodically thereafter. (5.10) • Immune reconstitution syndrome: May necessitate further evaluation and -----------------------DOSAGE AND ADMINISTRATION---------------------- treatment. (5.11) • SUSTIVA should be taken orally once daily on an empty stomach, • Redistribution/accumulation of body fat: Observed in patients receiving preferably at bedtime. (2) antiretroviral therapy. (5.12, 17) • Recommended adult dose: 600 mg. -

Psychotropic Drugs: Sedatives/Hypnotics, Antidepressants, and Antipsychotics
PSYCHOTROPIC DRUGS: SEDATIVES/HYPNOTICS, ANTIDEPRESSANTS, AND ANTIPSYCHOTICS INSTIs NNRTIs PIs RTI xBICTEGRAVIR xELVITEGRAVIR/ x DORAVIRINE x EFAVIRENZ xATAZANAVIR • TENOFOVIR • TENOFOVIR (Biktarvy) COBICISTAT (Pifeltro, (Sustiva, Atripla) (Reyataz/Norvir, ALAFENAMIDE, DISOPROXIL, TDF (Stribild, Genvoya) Delstrigo) Evotaz) TAF (Descovy, (Viread,Truvada, DOLUTEGRAVIR ETRAVIRINE x x Biktarvy, Genvoya, Atripla, Complera, (Tivicay, Triumeq, RILPIVIRINE (Intelence) DARUNAVIR x x Odefsey, Symtuza) Delstrigo, Stribild) Juluca) (Edurant, (Prezista/Norvir, x NEVIRAPINE Complera, Prezcobix, x RALTEGRAVIR (Viramune) •ABACAVIR (Kivexa, Odefsey, Juluca) Symtuza) (Isentress) Ziagen, Triumeq) xLOPINAVIR (Kaletra) SEDATIVES/HYPNOTICS xLorazepam, oxazepam, temazepam xAlprazolam, Potential for n Potential for p Potential for n bromazepam, benzodiazepine benzodiazepine benzodiazepine buspirone, clonazepam, estazolam, flurazepam, diazepam, nitrazepam, zolpidem, zopiclone xMidazolam, Potential for n Potential for p Potential for n triazolam benzodiazepine benzodiazepine benzodiazepine PSYCHOTROPICS INSTIs NNRTIs PIs RTI xBICTEGRAVIR xELVITEGRAVIR/ x DORAVIRINE x EFAVIRENZ xATAZANAVIR • TENOFOVIR • TENOFOVIR (Biktarvy) COBICISTAT (Pifeltro, (Sustiva, Atripla) (Reyataz/Norvir, ALAFENAMIDE, DISOPROXIL, TDF (Stribild, Genvoya) Delstrigo) Evotaz) TAF (Descovy, (Viread,Truvada, DOLUTEGRAVIR ETRAVIRINE x x Biktarvy, Genvoya, Atripla, Complera, (Tivicay, Triumeq, RILPIVIRINE (Intelence) DARUNAVIR x x Odefsey, Symtuza) Delstrigo, Stribild) Juluca) (Edurant, (Prezista/Norvir, -

Download PDF Flyer
REVIEWS IN PHARMACEUTICAL & BIOMEDICAL ANALYSIS Editors: Constantinos K. Zacharis and Paraskevas D. Tzanavaras eBooks End User License Agreement Please read this license agreement carefully before using this eBook. Your use of this eBook/chapter constitutes your agreement to the terms and conditions set forth in this License Agreement. Bentham Science Publishers agrees to grant the user of this eBook/chapter, a non-exclusive, nontransferable license to download and use this eBook/chapter under the following terms and conditions: 1. This eBook/chapter may be downloaded and used by one user on one computer. The user may make one back-up copy of this publication to avoid losing it. The user may not give copies of this publication to others, or make it available for others to copy or download. For a multi-user license contact [email protected] 2. All rights reserved: All content in this publication is copyrighted and Bentham Science Publishers own the copyright. You may not copy, reproduce, modify, remove, delete, augment, add to, publish, transmit, sell, resell, create derivative works from, or in any way exploit any of this publication’s content, in any form by any means, in whole or in part, without the prior written permission from Bentham Science Publishers. 3. The user may print one or more copies/pages of this eBook/chapter for their personal use. The user may not print pages from this eBook/chapter or the entire printed eBook/chapter for general distribution, for promotion, for creating new works, or for resale. Specific permission must be obtained from the publisher for such requirements. -
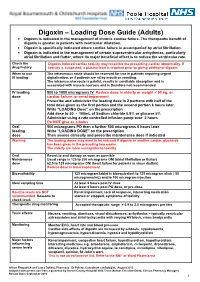
Digoxin – Loading Dose Guide (Adults) Digoxin Is Indicated in the Management of Chronic Cardiac Failure
Digoxin – Loading Dose Guide (Adults) Digoxin is indicated in the management of chronic cardiac failure. The therapeutic benefit of digoxin is greater in patients with ventricular dilatation. Digoxin is specifically indicated where cardiac failure is accompanied by atrial fibrillation. Digoxin is indicated in the management of certain supraventricular arrhythmias, particularly atrial fibrillation and flutter, where its major beneficial effect is to reduce the ventricular rate. Check the Digoxin-induced cardiac toxicity may resemble the presenting cardiac abnormality. If drug history toxicity is suspected, a plasma level is required prior to giving additional digoxin. When to use The intravenous route should be reserved for use in patients requiring urgent IV loading digitalisation, or if patients are nil by mouth or vomiting. The intramuscular route is painful, results in unreliable absorption and is associated with muscle necrosis and is therefore not recommended. IV loading 500 to 1000 micrograms IV Reduce dose in elderly or weight < 50 kg, or dose cardiac failure, or renal impairment Prescribe and administer the loading dose in 2 portions with half of the total dose given as the first portion and the second portion 6 hours later. Write “LOADING Dose” on the prescription Add dose to 50 - 100mL of Sodium chloride 0.9% or glucose 5% Administer using a rate controlled infusion pump over 2 hours Do NOT give as a bolus Oral 500 micrograms PO then a further 500 micrograms 6 hours later loading Write “LOADING DOSE” on the prescription dose Then assess clinically and prescribe maintenance dose if indicated Warning The loading doses may need to be reduced if digoxin or another cardiac glycoside has been given in the preceding two weeks. -

Guidelines for Use of Digoxin (Lanoxin )
Guidelines for use of Digoxin (Lanoxin) Recommended Neonatal Dose, Route, and Interval Loading or digitalizing dose: Total Loading Dose PMA (Weeks) IV (mcg/kg) PO (mcg/kg) 29 15 20 30 to 36 20 25 37 to 48 30 40 49 40 50 Divide into 3 doses over 24 hours Generally given in the following manner: 1. One-half the loading dose given immediately IV or PO 2. One-fourth the loading dose given 8 to 12 hours later IV or PO 3. The remaining one-fourth loading dose given after an additional 8 to 12 hours IV or PO 4. Administer IV slow push over 5 to 10 minutes 5. Obtain ECG 6 hours after digitalizing dose to assess for toxicity Maintenance dose: should be started 12 hours after the loading dose is completed Maintenance Dose PMA (Weeks) IV (mcg/kg) PO (mcg/kg) Interval (hours) 29 4 5 24 30 to 36 5 6 24 37 to 48 4 5 12 49 5 6 12 Titrate based on clinical response Chief Indications 1. Heart failure caused by diminished myocardial contractility 2. Supraventricular tachycardia, atrial flutter, atrial fibrillation Possible Adverse Reactions 1. Atrial or ventricular arrhythmias are common and may be an early indication of overdosage 2. Feeding intolerance, vomiting, diarrhea 3. Hypokalemia is associated with chronic Digoxin toxicity 4. Bradycardia due to depression of A-V conduction 5. Diuresis from improved cardiac output 6. Lethargy or seizures with toxicity Contraindications & Precautions 1. CAUTION use with pre-existing hypokalemia may lead to adverse reactions 2. CAUTION use with Indomethacin - may inhibit excretion of Digoxin 3. -

Disproportionality Analysis of Antiretrovirals
Conference on Retroviruses and Opportunistic Infections (CROI), Boston, Massachusetts, March 3–6 2014 Poster #761 Disproportionality Analysis of Antiretrovirals with Suicidality using FDA Adverse Event Reporting System (FAERS) Data Daniel Seekins Andrew Napoli1, John Coumbis2, Jennifer Wood2, Amit Soitkar2, Daniel Seekins1 Bristol-Myers Squibb, Plainsboro, NJ, USA 1Bristol-Myers Squibb, Plainsboro, NJ, USA, 2Bristol-Myers Squibb, Hopewell, NJ, USA Email: [email protected] INTRODUCTION Statistical Analysis Suicide Attempt CONSIDERATIONS n A disproportionality analysis was performed for the selected drug and selected AE using the MGPS method Efavirenz (n = 107) Antiretrovirals n Psychiatric events, including depression, suicidal ideation, suicide attempts, and completed Antidepressants Strengths suicide have been reported in patients receiving efavirenz since 19981 Etravirine (n = 4) n The disproportionality measure, Empirical Bayesian Geometric Mean (EBGM), and the EB05 n FAERS is a large, independent, public dataset containing real-world data that can enable corresponding 90% CI (EB05, EB95) were estimated Nevirapine (n = 41) EBGM n Recently, a pooled analysis of four AIDS Clinical Trials Group (ACTG) studies (A5095, A5142, EB95 identification of potential signals for rare AEs A5175, A5202), identified an increased rate of suicidality with efavirenz-containing regimens n The threshold score above which a drug-event disproportionality signal is likely to be present Atazanavir (n = 13) compared to efavirenz-free regimens: is an EB05