Trypanosomatids, Bacteria, and Viruses Potential Vectors?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Trabajo Fin De Grado Biotecnología
FACULTAD DE CIENCIA Y TECNOLOGÍA. LEIOA TRABAJO FIN DE GRADO BIOTECNOLOGÍA CLONACIÓN, EXPRESIÓN Y PURIFICACIÓN DE LA PROTEÍNA VIRAL VP4 DE TRIATOMA VIRUS Alumno: Goikolea Egia, Julen Fecha: Junio 2014 Director: Curso Académico: Dr. Diego Marcelo Guérin Aguilar 2013/14 ÍNDICE 1. INTRODUCCIÓN Y OBJETIVOS ........................................................................ 1 1.1. Ausencia de la proteína viral VP4 en la estructura cristalográfica de la cápside ............................................................................................................. 4 1.2. Viroporinas .................................................................................................... 5 1.3. Mecanismos de acción de proteínas con actividad permeabilizadora de membrana ........................................................................................................ 8 1.4. Objetivos ........................................................................................................ 11 2. DESARROLLO ..................................................................................................... 12 2.1. Materiales y Métodos ...................................................................................... 12 2.1.1. Purificación de TrV .............................................................................. 12 2.1.2. Extracción del ARN ............................................................................. 12 2.1.3. Clonaje de VP4-pET28a ...................................................................... -

Virology Journal Biomed Central
Virology Journal BioMed Central Research Open Access The use of RNA-dependent RNA polymerase for the taxonomic assignment of Picorna-like viruses (order Picornavirales) infecting Apis mellifera L. populations Andrea C Baker* and Declan C Schroeder Address: Marine Biological Association, The Laboratory, Citadel Hill, Plymouth, PL1 2PB, UK Email: Andrea C Baker* - [email protected]; Declan C Schroeder - [email protected] * Corresponding author Published: 22 January 2008 Received: 19 November 2007 Accepted: 22 January 2008 Virology Journal 2008, 5:10 doi:10.1186/1743-422X-5-10 This article is available from: http://www.virologyj.com/content/5/1/10 © 2008 Baker and Schroeder; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Background: Single-stranded RNA viruses, infectious to the European honeybee, Apis mellifera L. are known to reside at low levels in colonies, with typically no apparent signs of infection observed in the honeybees. Reverse transcription-PCR (RT-PCR) of regions of the RNA-dependent RNA polymerase (RdRp) is often used to diagnose their presence in apiaries and also to classify the type of virus detected. Results: Analysis of RdRp conserved domains was undertaken on members of the newly defined order, the Picornavirales; focusing in particular on the amino acid residues and motifs known to be conserved. Consensus sequences were compiled using partial and complete honeybee virus sequences published to date. -

Vectors of Chagas Disease, and Implications for Human Health1
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Denisia Jahr/Year: 2006 Band/Volume: 0019 Autor(en)/Author(s): Jurberg Jose, Galvao Cleber Artikel/Article: Biology, ecology, and systematics of Triatominae (Heteroptera, Reduviidae), vectors of Chagas disease, and implications for human health 1095-1116 © Biologiezentrum Linz/Austria; download unter www.biologiezentrum.at Biology, ecology, and systematics of Triatominae (Heteroptera, Reduviidae), vectors of Chagas disease, and implications for human health1 J. JURBERG & C. GALVÃO Abstract: The members of the subfamily Triatominae (Heteroptera, Reduviidae) are vectors of Try- panosoma cruzi (CHAGAS 1909), the causative agent of Chagas disease or American trypanosomiasis. As important vectors, triatomine bugs have attracted ongoing attention, and, thus, various aspects of their systematics, biology, ecology, biogeography, and evolution have been studied for decades. In the present paper the authors summarize the current knowledge on the biology, ecology, and systematics of these vectors and discuss the implications for human health. Key words: Chagas disease, Hemiptera, Triatominae, Trypanosoma cruzi, vectors. Historical background (DARWIN 1871; LENT & WYGODZINSKY 1979). The first triatomine bug species was de- scribed scientifically by Carl DE GEER American trypanosomiasis or Chagas (1773), (Fig. 1), but according to LENT & disease was discovered in 1909 under curi- WYGODZINSKY (1979), the first report on as- ous circumstances. In 1907, the Brazilian pects and habits dated back to 1590, by physician Carlos Ribeiro Justiniano das Reginaldo de Lizárraga. While travelling to Chagas (1879-1934) was sent by Oswaldo inspect convents in Peru and Chile, this Cruz to Lassance, a small village in the state priest noticed the presence of large of Minas Gerais, Brazil, to conduct an anti- hematophagous insects that attacked at malaria campaign in the region where a rail- night. -

Capsid Protein Identification and Analysis of Mature Triatoma
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Virology 409 (2011) 91–101 Contents lists available at ScienceDirect Virology journal homepage: www.elsevier.com/locate/yviro Capsid protein identification and analysis of mature Triatoma virus (TrV) virions and naturally occurring empty particles Jon Agirre a,c, Kerman Aloria b, Jesus M. Arizmendi c, Ibón Iloro d, Félix Elortza d, Rubén Sánchez-Eugenia a,h, Gerardo A. Marti e, Emmanuelle Neumann f, Félix A. Rey g, Diego M.A. Guérin a,c,h,⁎ a Unidad de Biofísica (CSIC-UPV/EHU), Barrio Sarriena S/N, 48940, Leioa, Bizkaia, Spain b Servicio General de Proteómica (SGIker), Universidad del País Vasco (UPV/EHU), 48940 Leioa, Spain c Departamento de Bioquímica y Biologia Molecular, Universidad del País Vasco (UPV/EHU), 48940 Leioa, Spain d CIC-BioGUNE, Proteomic plataform, CIBERehd, Proteored, Parque Tecnológico Bizkaia, Ed800, 48160 Derio, Bizkaia, Spain e Centro de Estudios Parasitológicos y de Vectores (CEPAVE) and Centro Regional de Investigaciones Científicas y Transferencia Tecnológicas La Rioja (CRILAR), 2#584 (1900) La Plata, Argentina f Laboratoire de Microscopie Electronique Structurale, Institut de Biologie Structurale Jean-Pierre Ebel, UMR 5075 CEA–CNRS–UJF, 41, rue Jules Horowitz, F-38027 Grenoble, Cedex 1, France g Unité de Virologie Structurale, CNRS URA 3015, Départment de Virologie, Institut Pasteur, 25 rue du Docteur Roux, 75724 Paris Cedex 15, France h Fundación Biofísica Bizkaia, B Sarriena S/N, 48940 Leioa, Bizkaia, Spain article info abstract Article history: Triatoma virus (TrV) is a non-enveloped +ssRNA virus belonging to the insect virus family Dicistroviridae. -

Bioenvironmental Correlates of Chagas´ Disease- S.I Curto
MEDICAL SCIENCES - Vol.I - Bioenvironmental Correlates of Chagas´ Disease- S.I Curto BIOENVIRONMENTAL CORRELATES OF CHAGAS´ DISEASE S.I Curto National Council for Scientific and Technological Research, Institute of Epidemiological Research, National Academy of Medicine – Buenos Aires. Argentina. Keywords: Chagas´ Disease, disease environmental factors, ecology of disease, American Trypanosomiasis. Contents 1. Introduction 2. Biological members and transmission dynamics of disease 3. Interconnection of wild, peridomestic and domestic cycles 4. Dynamics of domiciliation of the vectors: origin and diffusion of the disease 5. The rural housing as environmental problem 6. Bioclimatic factors of Triatominae species 7. Description of disease 8. Rates of human infection 9. Conclusions Summary Chagas´ disease is intimately related to sub development conditions of vast zones of Latin America. For that reason we must approach this problem through a transdisciplinary and totalized perspective. In that way we analyze the dynamics of different transmission cycles: wild, yard, domiciled, rural and also urban. The passage from a cycle to another constitutes a domiciliation dynamics even present that takes from hundred to thousands of years and that even continues in the measure that man destroys the natural ecosystems and eliminates the wild habitats where the parasite, the vectors and the guests take refuge. Also are analyzed the main physical factors that influence the persistence of the illness and their vectors as well as the peculiarities of the rural house of Latin America that facilitate the coexistence in time and man's space, the parasites and the vectors . Current Chagas’UNESCO disease transmission is –relate EOLSSd to human behavior, traditional, cultural and social conditions SAMPLE CHAPTERS 1. -

In Triatoma Infestans Known That the Entry of One Microorganism Favours the Entry of Another (Heteroptera : Reduviidae) from the Gran Chaco
Journal of Invertebrate Pathology 150 (2017) 101–105 Contents lists available at ScienceDirect Journal of Invertebrate Pathology journal homepage: www.elsevier.com/locate/jip Can Triatoma virus inhibit infection of Trypanosoma cruzi (Chagas, 1909) in T Triatoma infestans (Klug)? A cross infection and co-infection study ⁎ Gerardo Aníbal Martia,d, , Paula Ragoneb, Agustín Balsalobrea,d, Soledad Ceccarellia,d, María Laura Susevicha,d, Patricio Diosqueb, María Gabriela Echeverríac,d, Jorge Eduardo Rabinovicha,d a Centro de Estudios Parasitológicos y de Vectores (CEPAVE-CCT-La Plata-CONICET-UNLP), Boulevard 120 e/61 y 62, 1900 La Plata, Argentina b Unidad de Epidemiología Molecular del Instituto de Patología Experimental, Facultad de Ciencias de la Salud, Universidad Nacional de Salta, Salta, Argentina c Cátedra de Virología, Facultad de Ciencias Veterinarias (UNLP), 60 y 118, 1900 La Plata, Argentina d CCT-La Plata, 8#1467, 1900 La Plata, Argentina ARTICLE INFO ABSTRACT Keywords: Triatoma virus occurs infecting Triatominae in the wild (Argentina) and in insectaries (Brazil). Pathogenicity of Associated microorganisms Triatoma virus has been demonstrated in laboratory; accidental infections in insectaries produce high insect Dicistroviridae mortality. When more than one microorganism enters the same host, the biological interaction among them Protozoan differs greatly depending on the nature and the infection order of the co-existing species of microorganisms. We studied the possible interactions between Triatoma virus (TrV) and Trypanosoma cruzi (the etiological agent of Chagas disease) in three different situations: (i) when Triatoma virus is inoculated into an insect host (Triatoma infestans) previously infected with T. cruzi, (ii) when T. cruzi is inoculated into T. -

Kissing Bug” — Delaware, 2018 the United States, but Only a Few Cases of Chagas Disease from Paula Eggers1; Tabatha N
Morbidity and Mortality Weekly Report Notes from the Field Identification of a Triatoma sanguisuga Chagas disease is found. Triatomine bugs also are found in “Kissing Bug” — Delaware, 2018 the United States, but only a few cases of Chagas disease from Paula Eggers1; Tabatha N. Offutt-Powell1; Karen Lopez2; contact with the bugs have been documented in this country Susan P. Montgomery3; Gena G. Lawrence3 (2). Although presence of the vector has been confirmed in Delaware, there is no current evidence of T. cruzi in the state In July 2018, a family from Kent County, Delaware con- (2). T. cruzi is a zoonotic parasite that infects many mammal tacted the Delaware Division of Public Health (DPH) and species and is found throughout the southern half of the United the Delaware Department of Agriculture (DDA) to request States (2). Even where T. cruzi is circulating, not all triatomine assistance identifying an insect that had bitten their child’s face bugs are infected with the parasite. The likelihood of human while she was watching television in her bedroom during the T. cruzi infection from contact with a triatomine bug in the late evening hours. The parents were concerned about possible United States is low, even when the bug is infected (2). disease transmission from the insect. Upon investigation, DPH Precautions to prevent house triatomine bug infestation learned that the family resided in an older single-family home include locating outdoor lights away from dwellings such as near a heavily wooded area. A window air conditioning unit homes, dog kennels, and chicken coops and turning off lights was located in the bedroom where the bite occurred. -

Kissing Bugs: Not So Romantic
W 957 Kissing Bugs: Not So Romantic E. Hessock, Undergraduate Student, Animal Science Major R. T. Trout Fryxell, Associate Professor, Department of Entomology and Plant Pathology K. Vail, Professor and Extension Specialist, Department of Entomology and Plant Pathology What Are Kissing Bugs? Pest Management Tactics Kissing bugs (Triatominae), also known as cone-nosed The main goal of kissing bug management is to disrupt bugs, are commonly found in Central and South America, environments that the insects will typically inhabit. and Mexico, and less frequently seen in the southern • Focus management on areas such as your house, United States. housing for animals, or piles of debris. These insects are called “kissing bugs” because they • Fix any cracks, holes or damage to your home’s typically bite hosts around the eyes and mouths. exterior. Window screens should be free of holes to Kissing bugs are nocturnal blood feeders; thus, people prevent insect entry. experience bites while they are sleeping. Bites are • Avoid placing piles of leaves, wood or rocks within 20 usually clustered on the face and appear like other bug feet of your home to reduce possible shelter for the bites, as swollen, itchy bumps. In some cases, people insect near your home. may experience a severe allergic reaction and possibly • Use yellow lights to minimize insect attraction to anaphylaxis (a drop in blood pressure and constriction the home. of airways causing breathing difculty, nausea, vomiting, • Control or minimize wildlife hosts around a property to skin rash, and/or a weak pulse). reduce additional food sources. Kissing bugs are not specifc to one host and can feed • See UT Extension publications W 658 A Quick on a variety of animals, such as dogs, rodents, reptiles, Reference Guide to Pesticides for Pest Management livestock and birds. -

Structure of the Triatoma Virus Capsid Crystallography ISSN 0907-4449
research papers Acta Crystallographica Section D Biological Structure of the Triatoma virus capsid Crystallography ISSN 0907-4449 Gae¨lle Squires,a‡§ Joan Pous,a‡} The members of the Dicistroviridae family are non-enveloped Received 3 December 2012 Jon Agirre,b,c‡ Gabriela S. Rozas- positive-sense single-stranded RNA (+ssRNA) viruses patho- Accepted 16 February 2013 Dennis,d,e Marcelo D. Costabel,e genic to beneficial arthropods as well as insect pests of medical f Gerardo A. Marti, Jorge importance. Triatoma virus (TrV), a member of this family, PDB Reference: TrV, 3nap Navaza,a‡‡ Ste´phane infects several species of triatomine insects (popularly named Bressanelli,a Diego M. A. kissing bugs), which are vectors for human trypanosomiasis, more commonly known as Chagas disease. The potential use Gue´rinb,c* and Felix A. Reya*§§ of dicistroviruses as biological control agents has drawn considerable attention in the past decade, and several viruses aLaboratoire de Virologie Mole´culaire et of this family have been identified, with their targets covering Structurale, CNRS, 1 Avenue de la Terrasse, honey bees, aphids and field crickets, among others. Here, the 91198 Gif-sur-Yvette CEDEX, France, crystal structure of the TrV capsid at 2.5 A˚ resolution is bFundacio´n Biofı´sica Bizkaia, Barrio Sarriena S/N, 48940 Leioa, Bizkaia (FBB), Spain, cUnidad reported, showing that as expected it is very similar to that of de Biofı´sica (UBF, CSIC, UPV/EHU), PO Box Cricket paralysis virus (CrPV). Nevertheless, a number of 644, 48080 Bilbao, Spain, dDepartamento de distinguishing structural features support the introduction of a Biologı´a, Bioquı´mica y Farmacia, U.N.S., new genus (Triatovirus; type species TrV) under the San Juan 670, (8000) Bahı´a Blanca, Argentina, Dicistroviridae family. -

Phasing of the Triatoma Virus Diffraction Data Using a Cryo-Electron Microscopy Reconstruction
Available online at www.sciencedirect.com Virology 375 (2008) 85–93 www.elsevier.com/locate/yviro Phasing of the Triatoma virus diffraction data using a cryo-electron microscopy reconstruction L.F. Estrozi a, E. Neumann a, G. Squires c, G. Rozas-Dennis e, M. Costabel f, ⁎ ⁎ F.A. Rey d, D.M.A. Guérin b, , J. Navaza a, a IBS, Institut de Biologie Structurale Jean-Pierre Ebel. CEA; CNRS; Université Joseph Fourier. 41 rue Jules Horowitz, F-38027 Grenoble, France b Unidad de Biofísica (UPV/EHU-CSIC), P.O. Box 644, E-48080 Bilbao, Spain c Institut Curie, 26, rue d'Ulm 75248 Paris Cedex 05, France d Institut Pasteur, 25 rue du Dr. Roux, F-75724 Paris, France e Departamento de Biología, Bioquímica y Farmacia, U.N.S., San Juan 670, (8000) Bahía Blanca, Argentina f Grupo de Biofísica, Departamento de Física U.N.S., Av. Alem 1253, (8000) Bahía Blanca, Argentina Received 27 September 2007; returned to author for revision 31 October 2007; accepted 6 December 2007 Available online 4 March 2008 Abstract The blood-sucking reduviid bug Triatoma infestans, one of the most important vector of American human trypanosomiasis (Chagas disease) is infected by the Triatoma virus (TrV). TrV has been classified as a member of the Cripavirus genus (type cricket paralysis virus) in the Dicistroviridae family. This work presents the three-dimensional cryo-electron microscopy (cryo-EM) reconstruction of the TrV capsid at about 25 Å resolution and its use as a template for phasing the available crystallographic data by the molecular replacement method. The main structural differences between the cryo-EM reconstruction of TrV and other two viruses, one from the same family, the cricket paralysis virus (CrPV) and the human rhinovirus 16 from the Picornaviridae family are presented and discussed. -

Triatoma Sanguisuga, Eastern Blood-Sucking Conenose Bug (Hemiptera: Reduviidae) Chris Carlton, Forest Huval and T.E
Triatoma sanguisuga, Eastern Blood-Sucking Conenose Bug (Hemiptera: Reduviidae) Chris Carlton, Forest Huval and T.E. Reagan Description a professional insect diagnostician. At least nine members Adults of the eastern blood-sucking conenose bug of the subfamily Triatominae occur in the U.S., most in the are relatively large insects three-quarters of an inch to genus Triatoma, with additional species in Central and South seven-eighths of an inch (18 to 22 mm) in body length. America. The eastern blood-sucking conenose bug, Triatoma The body shape and coloration of adults are distinctive. sanguisuga, is the most commonly encountered species The elongated head narrows toward the front and is in Louisiana, but it is not the only species that occurs in black with slender, six-segmented antennae and a sharp, the state. Of 130 Louisiana specimens in the Louisiana three-segmented beak that folds beneath the head when State Arthropod Museum, 126 are T. sanguisuga, three are the insect is not feeding. The triangular thorax is black Triatoma lecticularia and one is Triatoma gerstaekeri. These with a narrow orange insects are often referred to as “kissing bugs,” a name that is or pinkish-orange used for the entire family Triatominae. margin and a prominent, triangular black Life Cycle scutellum between The eastern conenose and other kissing bugs are the wing bases. The typically associated with mammal burrows, nests or forewings are folded other harborages of small mammals. They require a flat across the abdomen blood meal during each stage of development, thus the when at rest, and each close association with mammals. -
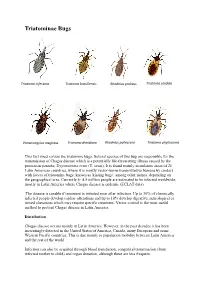
Triatominae Bugs
Triatominae Bugs Triatoma infestans Triatoma brasiliensis Rhodnius prolixus Triatoma sordida Panstrongylus megistus Triatoma dimidiata Rhodnius pallescens Triatoma phyllosoma This fact sheet covers the triatomine bugs. Several species of this bug are responsible for the transmission of Chagas disease which is a potentially life-threatening illness caused by the protozoan parasite, Trypanosoma cruzi (T. cruzi). It is found mainly in endemic areas of 21 Latin American countries, where it is mostly vector-borne transmitted to humans by contact with faeces of triatomine bugs, known as 'kissing bugs', among other names, depending on the geographical area. Currently 4- 4.5 million people are estimated to be infected worldwide, mostly in Latin America where Chagas disease is endemic (ECLAT data). The disease is curable if treatment is initiated soon after infection. Up to 30% of chronically infected people develop cardiac alterations and up to 10% develop digestive, neurological or mixed alterations which may require specific treatment. Vector control is the most useful method to prevent Chagas disease in Latin America. Distribution Chagas disease occurs mainly in Latin America. However, in the past decades it has been increasingly detected in the United States of America, Canada, many European and some Western Pacific countries. This is due mainly to population mobility between Latin America and the rest of the world. Infection can also be acquired through blood transfusion, congenital transmission (from infected mother to child) and organ donation, although these are less frequent. Transmission In Latin America, T. cruzi parasites are mainly transmitted by contact with the faeces of infected blood-sucking triatomine bugs. These bugs, vectors that carry the parasites, typically live in the cracks of poorly-constructed homes in rural or suburban areas.