Biological Role of Lactoferrin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Types of Acute Phase Reactants and Their Importance in Vaccination (Review)
BIOMEDICAL REPORTS 12: 143-152, 2020 Types of acute phase reactants and their importance in vaccination (Review) RAFAAT H. KHALIL1 and NABIL AL-HUMADI2 1Department of Biology, College of Science and Technology, Florida Agricultural and Mechanical University, Tallahassee, FL 32307; 2Office of Vaccines, Food and Drug Administration, Center for Biologics Evaluation and Research, Silver Spring, MD 20993, USA Received May 10, 2019; Accepted November 25, 2019 DOI: 10.3892/br.2020.1276 Abstract. Vaccines are considered to be one of the most human and veterinary medicine. Proteins which are expressed cost-effective life-saving interventions in human history. in the acute phase are potential biomarkers for the diagnosis The body's inflammatory response to vaccines has both of inflammatory disease, for example, acute phase proteins desired effects (immune response), undesired effects [(acute (APPs) are indicators of successful organ transplantation phase reactions (APRs)] and trade‑offs. Trade‑offs are and can be used to predict the ameliorative effect of cancer more potent immune responses which may be potentially therapy (1,2). APPs are primarily synthesized in hepatocytes. difficult to separate from potent acute phase reactions. The acute phase response is a spontaneous reaction triggered Thus, studying acute phase proteins (APPs) during vaccina- by disrupted homeostasis resulting from environmental distur- tion may aid our understanding of APRs and homeostatic bances (3). Acute phase reactions (APRs) usually stabilize changes which can result from inflammatory responses. quickly, after recovering from a disruption to homeostasis Depending on the severity of the response in humans, these within a few days to weeks; however, APPs expression levels reactions can be classified as major, moderate or minor. -

Lactoferrin and Its Detection Methods: a Review
nutrients Review Lactoferrin and Its Detection Methods: A Review Yingqi Zhang, Chao Lu and Jin Zhang * Department of Chemical and Biochemical Engineering, University of Western Ontario, London, ON N6A 5B9, Canada; [email protected] (Y.Z.); [email protected] (C.L.) * Correspondence: [email protected] Abstract: Lactoferrin (LF) is one of the major functional proteins in maintaining human health due to its antioxidant, antibacterial, antiviral, and anti-inflammatory activities. Abnormal levels of LF in the human body are related to some serious diseases, such as inflammatory bowel disease, Alzheimer’s disease and dry eye disease. Recent studies indicate that LF can be used as a biomarker for diagnosis of these diseases. Many methods have been developed to detect the level of LF. In this review, the biofunctions of LF and its potential to work as a biomarker are introduced. In addition, the current methods of detecting lactoferrin have been presented and discussed. We hope that this review will inspire efforts in the development of new sensing systems for LF detection. Keywords: lactoferrin; biomarkers; immunoassay; instrumental analysis; sensor 1. Introduction Lactoferrin (known as lactotransferrin, LF), with a molecular weight of about 80 kDa, is a functional glycoprotein, which contains about 690 amino acid residues. It was first isolated from bovine milk by Sorensen in 1939 and was first isolated from human milk by Citation: Zhang, Y.; Lu, C.; Zhang, J. Johanson in 1960 [1,2]. The three-dimensional structure of LF has been unveiled by high Lactoferrin and Its Detection resolution X-ray crystallographic analysis, and it consists of two homologous globular lobes Methods: A Review. -

Influence of Infection and Inflammation on Biomarkers of Nutritional Status
A2.4 INFLUENCE OF INFECTION AND INFLAMMATION ON BIOMARKERS OF NUTRITIONAL STATUS A2.4 Influence of infection and inflammation on biomarkers of nutritional status with an emphasis on vitamin A and iron David I. Thurnham1 and George P. McCabe2 1 Northern Ireland Centre for Food and Health, University of Ulster, Coleraine, United Kingdom of Great Britain and Northern Ireland 2 Statistics Department, Purdue University, West Lafayette, Indiana, United States of America Corresponding author: David I. Thurnham; [email protected] Suggested citation: Thurnham DI, McCabe GP. Influence of infection and inflammation on biomarkers of nutritional status with an emphasis on vitamin A and iron. In: World Health Organization. Report: Priorities in the assessment of vitamin A and iron status in populations, Panama City, Panama, 15–17 September 2010. Geneva, World Health Organization, 2012. Abstract n Many plasma nutrients are influenced by infection or tissue damage. These effects may be passive and the result of changes in blood volume and capillary permeability. They may also be the direct effect of metabolic alterations that depress or increase the concentration of a nutrient or metabolite in the plasma. Where the nutrient or metabolite is a nutritional biomarker as in the case of plasma retinol, a depression in retinol concentrations will result in an overestimate of vitamin A deficiency. In contrast, where the biomarker is increased due to infection as in the case of plasma ferritin concentrations, inflammation will result in an underestimate of iron deficiency. Infection and tissue damage can be recognized by their clinical effects on the body but, unfortunately, subclinical infection or inflammation can only be recognized by measur- ing inflammation biomarkers in the blood. -

The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria
Downloaded from orbit.dtu.dk on: Oct 02, 2021 The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria Kell, Douglas B.; Heyden, Eugene L.; Pretorius, Etheresia Published in: Frontiers in Immunology Link to article, DOI: 10.3389/fimmu.2020.01221 Publication date: 2020 Document Version Publisher's PDF, also known as Version of record Link back to DTU Orbit Citation (APA): Kell, D. B., Heyden, E. L., & Pretorius, E. (2020). The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria. Frontiers in Immunology, 11, [1221]. https://doi.org/10.3389/fimmu.2020.01221 General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. Users may download and print one copy of any publication from the public portal for the purpose of private study or research. You may not further distribute the material or use it for any profit-making activity or commercial gain You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. HYPOTHESIS AND THEORY published: 28 May 2020 doi: 10.3389/fimmu.2020.01221 The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria Douglas B. -

Serum Alpha2-Macroglobulin, Transferrin, Albumin, and Igg Levels in Preeclampsia
J. clin. Path., 1970, 23, 514-516 J Clin Pathol: first published as 10.1136/jcp.23.6.514 on 1 September 1970. Downloaded from Serum alpha2-macroglobulin, transferrin, albumin, and IgG levels in preeclampsia C. H. W. HORNE, P. W. HOWIE, AND R. B. GOUDIE From the University Departments ofPathology and Obstetrics and Gynaecology, Western Infirmary, Glasgow SYNOPSIS A radial immunodiffusion technique has been used to measure levels of four serum proteins in preeclampsia with or without proteinuria and in normal pregnant and non-pregnant controls. In preeclampsia unaccompanied by proteinuria, albumin and transferrin levels are similar to those found in the normal pregnant controls, but there are significant falls in 0x2-macroglobulin and IgG. When preeclampsia is accompanied by proteinuria there is a marked fall in albumin and an increase in o'2-macroglobulin. Since oU2-macroglobulin has antiplasmin activity it is possible that increased levels of this protein in preeclampsia accom-copyright. panied by proteinuria contribute to the intravascular coagulation which has been described in this disorder. Both in pregnancy and the nephrotic syndrome tension (bloodpressurehigher than 140/90mm Hg) increased levels of serum x2-macroglobulin have on two or more separate occasions after 28 weeks been reported (Schumacher and Schlumberger, of pregnancy in patients whose blood pressurehttp://jcp.bmj.com/ 1963; Schultze and Schwick, 1959). We therefore was less than 140/90 m-m Hg in the first trimester. thought it would be of interest to determine the Most of the patients had oedema. Preeclampsia serum oI2-macroglobulin levels in preeclampsia, a with proteinuria was diagnosed when proteinuria complication of pregnancy which bears a certain was detected for the first time after 28 weeks of similarity to the nephrotic syndrome. -

Weakness of Biochemical Markers of Nutritional and Inflammatory Status
European Journal of Clinical Nutrition (1997) 51, 148±153 ß 1997 Stockton Press. All rights reserved 0954±3007/97 $12.00 Weakness of biochemical markers of nutritional and in¯ammatory status as prognostic indices for growth retardation and morbidity of young children in central Africa R Tonglet1,4, E Mahangaiko Lembo2,4, M Dramaix3 and P Hennart3,4 1School of Public Health, Faculty of Medicine, Catholic University of Louvain, Brussels, Belgium; 2Rural Health District of Kirotshe, Goma, Northern Kivu, Zaire; 3School of Public Health, Faculty of Medicine, Free University of Brussels, Brussels, Belgium; and 4Centre Scienti®que et MeÂdical de l'Universite Libre de Bruxelles pour ses ActiviteÂs de CoopeÂration (CEMUBAC), Brussels, Belgium Objective: To determine to what extent biochemical markers of the nutritional and in¯ammatory status of young children are related to subsequent growth retardation and morbidity. Design: Population-based follow-up study of a cohort of children from admission to ®nal survey round six months later. Setting: Health area in Northern Kivu, Zaire. Subjects: 842 children under two years of age of whom about one-third gave informed consent to capillary blood collection. Main outcome measures: Concentration of albumin, transferrin, transthyretin, a1-acid glycoprotein, C-reactive protein, and complement component C3 at baseline, and three and six months later. Incremental growth per 1 month, 3 months and 6 months of follow-up. Cumulative incidence of disease per 1 month and 3 months interval. Results: A high proportion of children was with low concentrations of transport proteins and high concentrations of acute-phase reactants. Weight growth and arm circumference growth did not vary signi®cantly with respect to initial concentrations of biomarkers, but subsequent height growth was lower in children with high values of transferrin, a1-acid glycoprotein, and complement component C3 at baseline. -

Transferrin Plays a Central Role in Coagulation Balance by Interacting with Clotting Factors
www.nature.com/cr www.cell-research.com ARTICLE OPEN Transferrin plays a central role in coagulation balance by interacting with clotting factors Xiaopeng Tang1,2, Zhiye Zhang1, Mingqian Fang1,2, Yajun Han1, Gan Wang1, Sheng Wang3, Min Xue1,2, Yaxiong Li4, Li Zhang4, Jian Wu4, Biqing Yang5, James Mwangi1,2, Qiumin Lu1, Xiaoping Du6 and Ren Lai1,7,8,9,10 Coagulation balance is maintained through fine-tuned interactions among clotting factors, whose physiological concentrations vary substantially. In particular, the concentrations of coagulation proteases (pM to nM) are much lower than their natural inactivator antithrombin (AT, ~ 3 μM), suggesting the existence of other coordinators. In the current study, we found that transferrin (normal plasma concentration ~40 μM) interacts with fibrinogen, thrombin, factor XIIa (FXIIa), and AT with different affinity to maintain coagulation balance. Normally, transferrin is sequestered by binding with fibrinogen (normal plasma concentration ~10 μM) at a molar ratio of 4:1. In atherosclerosis, abnormally up-regulated transferrin interacts with and potentiates thrombin/FXIIa and blocks AT’s inactivation effect on coagulation proteases by binding to AT, thus inducing hypercoagulability. In the mouse model, transferrin overexpression aggravated atherosclerosis, whereas transferrin inhibition via shRNA knockdown or treatment with anti- transferrin antibody or designed peptides interfering with transferrin-thrombin/FXIIa interactions alleviated atherosclerosis. Collectively, these findings identify that transferrin -

Alpha -Antitrypsin Deficiency
The new england journal of medicine Review Article Dan L. Longo, M.D., Editor Alpha1-Antitrypsin Deficiency Pavel Strnad, M.D., Noel G. McElvaney, D.Sc., and David A. Lomas, Sc.D. lpha1-antitrypsin (AAT) deficiency is one of the most common From the Department of Internal Med genetic diseases. Most persons carry two copies of the wild-type M allele icine III, University Hospital RWTH of SERPINA1, which encodes AAT, and have normal circulating levels of the (Rheinisch–Westfälisch Technische Hoch A schule) Aachen, Aachen, Germany (P.S.); protein. Ninety-five percent of severe cases of AAT deficiency result from the homo- the Irish Centre for Genetic Lung Dis zygous substitution of a single amino acid, Glu342Lys (the Z allele), which is present ease, Royal College of Surgeons in Ire in 1 in 25 persons of European descent (1 in 2000 persons of European descent land, Beaumont Hospital, Dublin (N.G.M.); and UCL Respiratory, Division of Medi are homozygotes). Mild AAT deficiency typically results from a different amino cine, Rayne Institute, University College acid replacement, Glu264Val (the S allele), which is found in 1 in 4 persons in the London, London (D.A.L.). Address re Iberian peninsula. However, many other alleles have been described that have vari- print requests to Dr. Lomas at UCL Re spiratory, Rayne Institute, University Col able effects, such as a lack of protein production (null alleles), production of mis- lege London, London WC1E 6JF, United folded protein, or no effect on the level or function of circulating AAT (Table 1). Kingdom, or at d . -

Identification of Key Pathways and Genes in Dementia Via Integrated Bioinformatics Analysis
bioRxiv preprint doi: https://doi.org/10.1101/2021.04.18.440371; this version posted July 19, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Identification of Key Pathways and Genes in Dementia via Integrated Bioinformatics Analysis Basavaraj Vastrad1, Chanabasayya Vastrad*2 1. Department of Biochemistry, Basaveshwar College of Pharmacy, Gadag, Karnataka 582103, India. 2. Biostatistics and Bioinformatics, Chanabasava Nilaya, Bharthinagar, Dharwad 580001, Karnataka, India. * Chanabasayya Vastrad [email protected] Ph: +919480073398 Chanabasava Nilaya, Bharthinagar, Dharwad 580001 , Karanataka, India bioRxiv preprint doi: https://doi.org/10.1101/2021.04.18.440371; this version posted July 19, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract To provide a better understanding of dementia at the molecular level, this study aimed to identify the genes and key pathways associated with dementia by using integrated bioinformatics analysis. Based on the expression profiling by high throughput sequencing dataset GSE153960 derived from the Gene Expression Omnibus (GEO), the differentially expressed genes (DEGs) between patients with dementia and healthy controls were identified. With DEGs, we performed a series of functional enrichment analyses. Then, a protein–protein interaction (PPI) network, modules, miRNA-hub gene regulatory network and TF-hub gene regulatory network was constructed, analyzed and visualized, with which the hub genes miRNAs and TFs nodes were screened out. Finally, validation of hub genes was performed by using receiver operating characteristic curve (ROC) analysis. -
2019 Laboratory Report
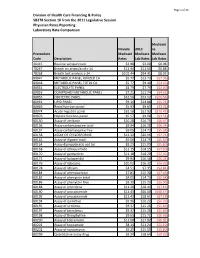
Page 1 of 24 Division of Health Care Financing & Policy SB278 Section 16 from the 2011 Legislative Session Physician Rates Reporting Laboratory Rate Comparison Medicaid Nevada 2019 vs. Proccedure Medicaid Medicare Medicare Code Description Rates Lab Rates Lab Rates 36415 Routine venipuncture $3.98 $3.00 $0.98 78267 Breath tst attain/anal c-14 $11.94 $11.06 $0.88 78268 Breath test analysis c-14 $102.44 $94.41 $8.03 80047 METABOLIC PANEL IONIZED CA $5.77 $13.73 ($7.96) 80048 METABOLIC PANEL TOTAL CA $5.77 $9.40 ($3.63) 80051 ELECTROLYTE PANEL $4.79 $7.79 ($3.00) 80053 COMPREHEN METABOLIC PANEL $7.21 $11.74 ($4.53) 80055 OBSTETRIC PANEL $32.56 $53.12 ($20.56) 80061 LIPID PANEL $9.14 $14.88 ($5.74) 80069 Renal function panel $5.93 $9.65 ($3.72) 80074 Acute hepatitis panel $32.50 $52.93 ($20.43) 80076 Hepatic function panel $5.57 $9.08 ($3.51) 80150 Assay of amikacin $10.28 $16.75 ($6.47) 80156 Assay carbamazepine total $9.94 $16.18 ($6.24) 80157 Assay carbamazepine free $9.05 $14.73 ($5.68) 80158 ASSAY OF CYCLOSPORINE $12.32 $20.06 ($7.74) 80162 Assay of digoxin total $9.06 $14.75 ($5.69) 80164 Assay dipropylacetic acd tot $9.25 $15.05 ($5.80) 80168 Assay of ethosuximide $11.15 $18.15 ($7.00) 80170 Assay of gentamicin $11.18 $18.20 ($7.02) 80173 Assay of haloperidol $9.94 $16.18 ($6.24) 80176 Assay of lidocaine $10.02 $16.32 ($6.30) 80178 Assay of lithium $4.51 $7.35 ($2.84) 80184 Assay of phenobarbital $7.81 $15.30 ($7.49) 80185 Assay of phenytoin total $9.05 $14.73 ($5.68) 80186 Assay of phenytoin free $9.39 $15.29 ($5.90) 80188 Assay of -

A Deficiency in Golgi Localised N-Acetyl-Glucosaminyltransferase II 125
Archives of Disease in Childhood 1994; 71: 123-127 123 Carbohydrate deficient glycoprotein syndrome type II: a deficiency in Golgi localised Arch Dis Child: first published as 10.1136/adc.71.2.123 on 1 August 1994. Downloaded from N-acetyl-glucosaminyltransferase II J Jaeken, H Schachter, H Carchon, P De Cock, B Coddeville, G Spik Abstract Case report The carbohydrate deficient glycoprotein The patient, a Belgian boy, was born in 1983 (CDG) syndromes are a family of genetic after a normal pregnancy and delivery. His multisystemic disorders with severe birth weight was 3250 g, length 50 cm, and nervous system involvement. This report head circumference 35 cm. He had a younger is on a child with a CDG syndrome that healthy brother; the parents were not related. differs from the classical picture but is The father's height was on the 3rd centile and very similar to a patient reported in head circumference on the 90th centile; he 1991. Both these patients are therefore showed some facial dysmorphism with a short designated CDG syndrome type II. neck but was otherwise normal. From birth Compared with type I patients they have the patient was hypotonic. He showed a more severe psychomotor retardation dysmorphic features: a hook nose, large but no peripheral neuropathy nor cere- dysplastic ears in oblique position, thin lips, bellar hypoplasia. The serum transferrin prognathia of the maxilla, short neck, isoform pattern obtained by isoelec- proximal implantation of the thumbs, and tric focusing showed disialotransferrin irregular position of the toes. There was a as the major fraction. The serum cardiac murmur due to a small ventricular disialotransferrin, studied in the present septal defect. -

The Acute Phase Response Is a Prominent Renal Proteome Change in Sepsis in Mice
International Journal of Molecular Sciences Article The Acute Phase Response Is a Prominent Renal Proteome Change in Sepsis in Mice Beáta Róka 1,Pál Tod 1,2, Tamás Kaucsár 1, Matej Vizovišek 3 , Robert Vidmar 3, Boris Turk 3,4 , Marko Fonovi´c 3,4,Gábor Szénási 1 and Péter Hamar 1,2,* 1 Institute of Translational Medicine, Semmelweis University, 1094 Budapest, Hungary; [email protected] (B.R.); [email protected] (P.T.); [email protected] (T.K.); [email protected] (G.S.) 2 Institute for Translational Medicine, Medical School, University of Pécs, 7624 Pécs, Hungary 3 Department of Biochemistry and Molecular and Structural Biology, Jožef Stefan Institute, 1000 Ljubljana, Slovenia; [email protected] (M.V.); [email protected] (R.V.); [email protected] (B.T.); [email protected] (M.F.) 4 Centre of Excellence for Integrated Approaches in Chemistry and Biology of Proteins, 1000 Ljubljana, Slovenia * Correspondence: [email protected]; Tel.: +36-20-825-9751; Fax: +36-1-210-0100 Received: 18 November 2019; Accepted: 20 December 2019; Published: 27 December 2019 Abstract: (1) Background: Sepsis-induced acute kidney injury (AKI) is the most common form of acute kidney injury (AKI). We studied the temporal profile of the sepsis-induced renal proteome changes. (2) Methods: Male mice were injected intraperitoneally with bacterial lipopolysaccharide (LPS) or saline (control). Renal proteome was studied by LC-MS/MS (ProteomeXchange: PXD014664) at the early phase (EP, 1.5 and 6 h after 40 mg/kg LPS) and the late phase (LP, 24 and 48 h after 10 mg/kg LPS) of LPS-induced AKI.