Notes of Ceramics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

10-1 CHAPTER 10 DEFORMATION 10.1 Stress-Strain Diagrams And
EN380 Naval Materials Science and Engineering Course Notes, U.S. Naval Academy CHAPTER 10 DEFORMATION 10.1 Stress-Strain Diagrams and Material Behavior 10.2 Material Characteristics 10.3 Elastic-Plastic Response of Metals 10.4 True stress and strain measures 10.5 Yielding of a Ductile Metal under a General Stress State - Mises Yield Condition. 10.6 Maximum shear stress condition 10.7 Creep Consider the bar in figure 1 subjected to a simple tension loading F. Figure 1: Bar in Tension Engineering Stress () is the quotient of load (F) and area (A). The units of stress are normally pounds per square inch (psi). = F A where: is the stress (psi) F is the force that is loading the object (lb) A is the cross sectional area of the object (in2) When stress is applied to a material, the material will deform. Elongation is defined as the difference between loaded and unloaded length ∆푙 = L - Lo where: ∆푙 is the elongation (ft) L is the loaded length of the cable (ft) Lo is the unloaded (original) length of the cable (ft) 10-1 EN380 Naval Materials Science and Engineering Course Notes, U.S. Naval Academy Strain is the concept used to compare the elongation of a material to its original, undeformed length. Strain () is the quotient of elongation (e) and original length (L0). Engineering Strain has no units but is often given the units of in/in or ft/ft. ∆푙 휀 = 퐿 where: is the strain in the cable (ft/ft) ∆푙 is the elongation (ft) Lo is the unloaded (original) length of the cable (ft) Example Find the strain in a 75 foot cable experiencing an elongation of one inch. -

Definition of Brittleness: Connections Between Mechanical
DEFINITION OF BRITTLENESS: CONNECTIONS BETWEEN MECHANICAL AND TRIBOLOGICAL PROPERTIES OF POLYMERS Haley E. Hagg Lobland, B.S., M.S. Dissertation Prepared for the Degree of DOCTOR OF PHILOSOPHY UNIVERSITY OF NORTH TEXAS August 2008 APPROVED: Witold Brostow, Major Professor Rick Reidy, Committee Member and Chair of the Department of Materials Science and Engineering Dorota Pietkewicz, Committee Member Nigel Shepherd, Committee Member Costas Tsatsoulis, Dean of the College of Engineering Sandra L. Terrell, Dean of the Robert B. Toulouse School of Graduate Studies Hagg Lobland, Haley E. Definition of brittleness: Connections between mechanical and tribological properties of polymers. Doctor of Philosophy (Materials Science and Engineering), August 2008, 51 pages, 5 tables, 13 illustrations, 106 references. The increasing use of polymer-based materials (PBMs) across all types of industry has not been matched by sufficient improvements in understanding of polymer tribology: friction, wear, and lubrication. Further, viscoelasticity of PBMs complicates characterization of their behavior. Using data from micro-scratch testing, it was determined that viscoelastic recovery (healing) in sliding wear is independent of the indenter force within a defined range of load values. Strain hardening in sliding wear was observed for all materials—including polymers and composites with a wide variety of chemical structures—with the exception of polystyrene (PS). The healing in sliding wear was connected to free volume in polymers by using pressure- volume-temperature (P-V-T) results and the Hartmann equation of state. A linear relationship was found for all polymers studied with again the exception of PS. The exceptional behavior of PS has been attributed qualitatively to brittleness. -

New Model of Brittleness Index to Locate the Sweet Spots for Hydraulic Fracturing in Unconventional Reservoirs
VOL. 14, NO. 18, SEPTEMBER 2019 ISSN 1819-6608 ARPN Journal of Engineering and Applied Sciences ©2006-2019 Asian Research Publishing Network (ARPN). All rights reserved. www.arpnjournals.com NEW MODEL OF BRITTLENESS INDEX TO LOCATE THE SWEET SPOTS FOR HYDRAULIC FRACTURING IN UNCONVENTIONAL RESERVOIRS Omer Iqbal1, Maqsood Ahmad1 and Eswaran Padmanabhan2 1Department of Petroleum Engineering, Institute of Hydrocarbon recovery (IHR), Universiti Teknologi PETRONAS, Malaysia 2Department of Petroleum Geoscience, Institute of Hydrocarbon recovery (IHR), Universiti Teknologi PETRONAS, Malaysia E-Mail: [email protected] ABSTRACT Rock characterization in term of brittleness is necessary for successful stimulation of shale gas reservoirs. High brittleness is required to prevent healing of natural and induced hydraulic fractures and also to decrease the breakdown pressure for fracture initiation and propagation. Several definitions of brittleness and methods for its estimation has been reviewed in this study in order to come up with most applicable and promising conclusion. The brittleness in term of brittleness index (BI) can be quantified from laboratory on core samples, geophysical methods and from well logs. There are many limitations in lab-based estimation of BI on core samples but still consider benchmark for calibration with other methods. The estimation of brittleness from mineralogy and dynamic elastic parameters like Young’s modulus, Poison’s ratio is common in field application. The new model of brittleness index is proposed based on mineral contents and geomechanical properties, which could be used to classify rock into brittle and ductile layers. The importance of mechanical behavior in term of brittle and ductile in shale gas fracturing were also reviewed because shale with high brittleness index (BI) or brittle shale exist natural fractures that are closed before stimulation and can provide fracture network or avenues through stimulation. -

Cohesive Crack with Rate-Dependent Opening and Viscoelasticity: I
International Journal of Fracture 86: 247±265, 1997. c 1997 Kluwer Academic Publishers. Printed in the Netherlands. Cohesive crack with rate-dependent opening and viscoelasticity: I. mathematical model and scaling ZDENEKÏ P. BAZANTÏ and YUAN-NENG LI Department of Civil Engineering, Northwestern University, Evanston, Illinois 60208, USA Received 14 October 1996; accepted in revised form 18 August 1997 Abstract. The time dependence of fracture has two sources: (1) the viscoelasticity of material behavior in the bulk of the structure, and (2) the rate process of the breakage of bonds in the fracture process zone which causes the softening law for the crack opening to be rate-dependent. The objective of this study is to clarify the differences between these two in¯uences and their role in the size effect on the nominal strength of stucture. Previously developed theories of time-dependent cohesive crack growth in a viscoelastic material with or without aging are extended to a general compliance formulation of the cohesive crack model applicable to structures such as concrete structures, in which the fracture process zone (cohesive zone) is large, i.e., cannot be neglected in comparison to the structure dimensions. To deal with a large process zone interacting with the structure boundaries, a boundary integral formulation of the cohesive crack model in terms of the compliance functions for loads applied anywhere on the crack surfaces is introduced. Since an unopened cohesive crack (crack of zero width) transmits stresses and is equivalent to no crack at all, it is assumed that at the outset there exists such a crack, extending along the entire future crack path (which must be known). -

Multidisciplinary Design Project Engineering Dictionary Version 0.0.2
Multidisciplinary Design Project Engineering Dictionary Version 0.0.2 February 15, 2006 . DRAFT Cambridge-MIT Institute Multidisciplinary Design Project This Dictionary/Glossary of Engineering terms has been compiled to compliment the work developed as part of the Multi-disciplinary Design Project (MDP), which is a programme to develop teaching material and kits to aid the running of mechtronics projects in Universities and Schools. The project is being carried out with support from the Cambridge-MIT Institute undergraduate teaching programe. For more information about the project please visit the MDP website at http://www-mdp.eng.cam.ac.uk or contact Dr. Peter Long Prof. Alex Slocum Cambridge University Engineering Department Massachusetts Institute of Technology Trumpington Street, 77 Massachusetts Ave. Cambridge. Cambridge MA 02139-4307 CB2 1PZ. USA e-mail: [email protected] e-mail: [email protected] tel: +44 (0) 1223 332779 tel: +1 617 253 0012 For information about the CMI initiative please see Cambridge-MIT Institute website :- http://www.cambridge-mit.org CMI CMI, University of Cambridge Massachusetts Institute of Technology 10 Miller’s Yard, 77 Massachusetts Ave. Mill Lane, Cambridge MA 02139-4307 Cambridge. CB2 1RQ. USA tel: +44 (0) 1223 327207 tel. +1 617 253 7732 fax: +44 (0) 1223 765891 fax. +1 617 258 8539 . DRAFT 2 CMI-MDP Programme 1 Introduction This dictionary/glossary has not been developed as a definative work but as a useful reference book for engi- neering students to search when looking for the meaning of a word/phrase. It has been compiled from a number of existing glossaries together with a number of local additions. -
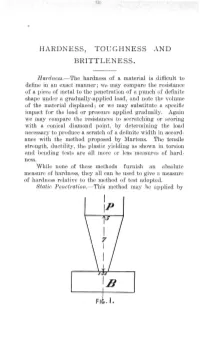
HARDNESS, Toeghness and BRITTLENESS. FI . I
HARDNESS, TOeGHNESS AND BRITTLENESS. H ardness.-The hardness of a material is difficult to define in an exact manner; we may compare the r esistance of a piece of metal to the penetration of a puncn of definite shape under a gradually-applied load, and note t he volume of the material displaced; or we may substitute a specifie impact for the load or pressure applied gradually. Again we may compare the resistances to secratching or scoring with a conical diamond point, by determining the load necessary to produce a scratch of a definite width in accord ance with the method proposed by Martens. The tensile strength, ductility, the plastic yielding as shown in torsion and bending tests are all more or less measures of hard ~ ness. While none of these methods furnish an absolute measure of hardness, they all can be used to give a measure of hardness relative to the method of test adopted. Static Penetration.- This method may be applied by I IP FI . I. HARDNESS, TOUGHNESS AND BRITTLENESS. 213 causing a punch, P, to penetrate a test piece B, fig. 1, under a gradually-increasing pressure. lVlessrs. Calvert and Johnson used a 'small lever machine to apply a definite pressure to the punch P , having th') dimensions shown, ex presesd in millimetres, and determined the load necessary to produce an indentation of 3.5 m.m. in the metal under . test in half an hour. The hardness of the metals, tested in this way, is expressed relatively to Staffordshire cast iron, r epresented by 1.000. -

Intercrystalline Brittleness of Lead
. INTERCRYSTALLINE BRITTLENESS OF LEAD By Henry S. Rawdon CONTENTS Page I. Introduction '. 215 II. Corroded lead sheathing. 216 III. The allotropism of lead 219 IV. Experimental embrittlement of lead 220 1 Immersion in solutions of lead salts 220 2. Electrolysis of lead in concentrated nitric acid 228 V. Explanation of results 231 VI. Summary 232 I. INTRODUCTION The relation between the course or the path of the fracture of metals and alloys produced in service or as a result of certain laboratory tests and the crystalline units of which such materials are composed is of utmost importance. The fracture of normal material is, in general, intracrystalline ; that is, it consists of a break across the grains rather than of a separation between them. An intercrystalline fracture indicates either that the metal is of very inferior quality or that the break occurred under very unusual conditions; for example, at a very high temperature. The usual mechanical tests, when applied to metals of the type which breaks with an intercrystalline fracture, merely measure the coherence of adjacent grains for one another and reveal little as to the real properties of the metal itself. Even such a soft, plastic substance as lead, under suitable con- ditions, may be rendered so weak and brittle that it can be easily crumbled into powder by the fingers, although the constituent grains have lost none of the intrinsic properties of lead. Various erroneous explanations of this behavior of lead have appeared in the scientific literature, the change being described usually as an allotropic one. The importance, in an industrial sense, of a proper explanation of this type of the corrosion of lead justifies the description of the type of metal deterioration which follows. -

Brittleness of Paper
LAPPEENRANTA UNIVERSITY OF TECHNOLOGY School of Technology LUT Chemistry Laboratory of Fiber and Paper Technology Perttu Muhonen BRITTLENESS OF PAPER Examiners: Professor Eeva Jernström Professor Kaj Backfolk Preface This Master’s thesis has been carried out at Lappeenranta University of Technology. Thesis was done for and financed by UPM-Kymmene Oyj Research Center in Lappeenranta. I would like to express my warmest thanks to the instructor of my thesis D.Sc. (tech.) Markku Ora for all his support, guidance and practical advice during this work and my supervising professors Eeva Jernström and Kaj Backfolk for their good advice. D.Sc. (tech.) Jouko Lehto and Mika Räty, thank you for taking part in the support group and for providing interesting aspects on my work. I would also like to thank Mr. Barry Madden for proof reading the Literature part of the thesis. I am also very grateful to the professional personnel at the laboratories of UPM Research Center and UPM Kaukas paper mill. I would like to show my gratitude to my family for their love, understanding and support through my studies. I would also like to thank my friends for all the unforgettable moments we have seen together. Special thanks I would like to dedicate to Paula: without your support, patience and love this work would never have been completed. Lappeenranta, November 2013 Perttu Muhonen ABSTRACT Lappeenranta University of Technology School of Technology LUT Chemistry Laboratory of Fiber and Paper Technology Perttu Muhonen BRITTLENESS OF PAPER Thesis for the Degree of Master of Science in Technology 2013 110 pages, 58 figures, 15 tables and 11 appendices Examiners: Professor Eeva Jernström Professor Kaj Backfolk Supervisor: D.Sc. -

Mechanical Properties of Brittle Material
MECHANICAL PROPERTIES OF BRITTLE MATERIAL L. R. Cornwell Department of Mechanical Engineering Texas A&M University College Station, Texas 77843-3 123 Telephone 409-845-125 1 367 $~EcED!WG PAGE DLAhlK tJ3T hiLk4ED Mechanical Properties of Brittle Materials L.R.Cornwell and H.R. Thornton Department of Mechanical Engineering Texas A&M University College Station, Texas 77843-3123 KEY WORDS: Brittle materials, bending modulus, wood, glass, surface cracks, fracture toughness PREREQUISITE KNOWLEDGE: This experiment is suitable for students who have been introduced to the concepts of ductiiity and brittleness. They should also understand the meaning of modulus of. elasticity. EQUIPMENT AND SUPPLIES: Universal testing machine such as Instron and MTS, bending fixture, rectangular wood specimens 1 crn by 1 crn 15 cm, glass slides. INTRODUCTION Brittle materials are difficuit to tensile test because of gripping problems; they either crack in conventional grips or are crushed. Furthermore they may be difficult to make into tensile specimens for example threaded ends or of dogbone shape. To overcome the problem simple rectangular shapes can be used in bending (i.e., a simple beam) in order to obtain the modulus of rupture and the elastic modulus. The equipment necessary consists of a fixture for supporting the specimens horizontally at two points, these contact points being rollers which are free to rotate. The force necessary to bend the specimen is produced by tup attached to the crosshead of an Instron machine. PROCEDURE The specimens are rectangular in shape and are measured for width and thickness; the length is the distance between the two supports. -

1-6 FRACTURING: BRITTLENESS 1-6.1 the IMPORTANCE of BRITTLENESS Most Ceramics Are Brittle at Room Temperature
ﺍﻟﻤﺮﺣﻠﺔ ﺍﻟﺜﺎﻟﺜﺔ/ﻓﺮﻉ ﺍﻟﺴﻴﺮﺍﻣﻴﻚ ﻭﻣﻮﺍﺩ ﺍﻟﺒﻨﺎﺀ Properties of Ceramic Materials 1-6 FRACTURING: BRITTLENESS 1-6.1 THE IMPORTANCE OF BRITTLENESS Most ceramics are brittle at room temperature. That is they fracture with very little plastic deformation. Many archeologists believe that our very existence depended on the brittleness of ceramics, particularly flint. The fracture of flint, like cubic zirconia, diamond, and glass, is termed conchoidal, producing shell-like fracture surfaces. These surfaces are very sharp and were utilized in early stone tools to cut and shape wood and to butcher animals required for food. The hides were used for clothing and were attached to wooden frames to make shelters. Stone tools were necessary to cut vegetation and cultivate plants, allowing a change from a food- gathering economy to one of food production. This revolutionary change from hunting to farming laid the foundation for civilization. Bulletproof glass, which is a laminate of glass and polycarbonate, is a dramatic illustration of the utilization of brittle fracture. The glass absorbs the energy of the projectile either in elastic changes or ultimately in the creation of new surfaces when it fractures. The polymer provides an additional mechanism for absorption of energy before the projectile exits the final layer. The brittle behavior of ceramics is critical to the successful operation of ceramic armor. In addition to brittleness the basic requirements are that it be - Hard - Lightweight The first ceramic used in this application was alumina backed with a laminate of fiberglass and polyester resin called “Doron.” Boron carbide (B4C) ceramic armor. Brittle fracture is used for shaping and machining ceramics after they have been fired. -

Brittle Failure and Low-Temperature Welding
Brittle Failure and /:" . Low-Temperature Welding , c Downloaded from http://onepetro.org/jcpt/article-pdf/8/01/35/2166420/petsoc-69-01-06.pdf by guest on 30 September 2021 ,;,.'.:-. K. WINTERTON Head, Welding Section, Physical Metallu1'gy Divisicn, Mines B,'ancl!, Ottawa , 'J : .'- '., .! .,:, J ABSTRACT INTRODUCTION , :) The causes of brittle failure are explained, and the THERE ARE ALL KINDS OF TROUBLES that can develop aspects of crack propagation and crack initiation treated in equipment in northern climates. These may result separatel)·. The selection of steels for service at low temperatures is considered, and some instances are given from improper selection of materials, unsuitable over of special code requirements. General advice is presented all design, inappropriate design or choice of particular for the minimization of the problems of brittle failure. components, manufacturing defects with special sig 'Velding in cold weather affects personnel, materials and nificance for operation at low temperatures, etc. equipment. Metallurgieal factors are also involved. as revealed by experimentation and practical experience_ It is not surprising that suitable equipment and Code requirements are explained. Practical advice is materials could in most cases be provided, but that given for overcoming the problems that may be en countered. this would entail unwelcome increases in costs. More over, in Canad~l at least, the problems have been at tacked piecemeal, and there is no systematic ap proach available to those who are not immediate!)' deterred by the prospect of paying more for what the}" need. At the University of Alaska, the engineer ing faculty places emphasis on special training of, students in the unique aspects of work in the North. -

Enghandbook.Pdf
785.392.3017 FAX 785.392.2845 Box 232, Exit 49 G.L. Huyett Expy Minneapolis, KS 67467 ENGINEERING HANDBOOK TECHNICAL INFORMATION STEELMAKING Basic descriptions of making carbon, alloy, stainless, and tool steel p. 4. METALS & ALLOYS Carbon grades, types, and numbering systems; glossary p. 13. Identification factors and composition standards p. 27. CHEMICAL CONTENT This document and the information contained herein is not Quenching, hardening, and other thermal modifications p. 30. HEAT TREATMENT a design standard, design guide or otherwise, but is here TESTING THE HARDNESS OF METALS Types and comparisons; glossary p. 34. solely for the convenience of our customers. For more Comparisons of ductility, stresses; glossary p.41. design assistance MECHANICAL PROPERTIES OF METAL contact our plant or consult the Machinery G.L. Huyett’s distinct capabilities; glossary p. 53. Handbook, published MANUFACTURING PROCESSES by Industrial Press Inc., New York. COATING, PLATING & THE COLORING OF METALS Finishes p. 81. CONVERSION CHARTS Imperial and metric p. 84. 1 TABLE OF CONTENTS Introduction 3 Steelmaking 4 Metals and Alloys 13 Designations for Chemical Content 27 Designations for Heat Treatment 30 Testing the Hardness of Metals 34 Mechanical Properties of Metal 41 Manufacturing Processes 53 Manufacturing Glossary 57 Conversion Coating, Plating, and the Coloring of Metals 81 Conversion Charts 84 Links and Related Sites 89 Index 90 Box 232 • Exit 49 G.L. Huyett Expressway • Minneapolis, Kansas 67467 785-392-3017 • Fax 785-392-2845 • [email protected] • www.huyett.com INTRODUCTION & ACKNOWLEDGMENTS This document was created based on research and experience of Huyett staff. Invaluable technical information, including statistical data contained in the tables, is from the 26th Edition Machinery Handbook, copyrighted and published in 2000 by Industrial Press, Inc.