Ethical Perspectives in Biogerontology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biogerontology: a Novel Tool to Stay Healthy in Old Age
MoPAct Policy Brief: 5 Biogerontology: a novel tool to stay healthy in old age Policy priority Healthy lifestyle interventions in particular regarding nutrition and vaccination need to be implemented early in life with a lifecourse perspective. Summary: Key findings: • Accumulating evidence from experimental studies shows that aging is not inevitably linked with the development of chronic diseases. • Only 20-25% of healthy life expectancy (HLE) is predetermined by genes; lifestyle and environment play a major role. • Age-associated accumulation of molecular and cellular damage can be prevented or greatly delayed by lifestyle interventions e.g. dietary manipulations. • Classical strategies (e.g. nutrition, exercise, vaccination) require broad communication to public. • Novel strategies (e.g. dietary interventions, novel drugs, stem cells) need successful translation from the understanding of molecular mechanism to animal models to clinic. • To be successful interventions need to be started early Figure 1. Strategies to increase HLE. (1) Classical interventions include nutrition, exercise, vaccination, no smoking/alcohol/drugs. (2) Novel interventions include in life with a life-course perspective. dietary interventions, clearance of senescent and damaged cells, mitohormetics, stem cells, drugs against inflammation, rejuvenation factors from blood, telomeres, Background: epigenetic drugs, chaperons and proteolytic systems (novel interventions adapted from López-Otín et al., Cell 2013). Population aging is progressing fast in all developed countries. Aging is associated with the development of multiple serious chronic illnesses, including type 2 diabetes, hypertension, heart Prevention disease, stroke, cancer, cognitive impairment and increased Prevention prior to the development of age-associated diseases morbidity and mortality from infectious diseases. As people is key for successful aging. -

Chapter 6: Old People Are People Too, So Let's Act
Career Planning & Adult Development JOURNAL Volume 31, Number 2 ISSN 0736-1920 Summer 2015 OUR FUTURE: Projections of Work and Life Helen Harkness, Guest Editor QThe Future Q The End of Work as We Know It QJobs and Careers on the Front Line of the Future QSilicon Valley and the New Rules of Work Q Training Challenges Facing Education and Training and Career Development in the Future QOld people are people too, so let’s act accordingly Q Crisis of Human Capital in Aerospace: It’s All About the STEM QCollege for All – Reality or Flawed Myth? QOur Jobs: The American Workforce and Economy in Crisis Q The Future Has Arrived: The Future is Now the Present Summer 2015..............................Career Planning and Adult Development JOURNAL..............................1 CAREER PLANNING and ADULT DEVELOPMENT JOURNAL Volume 31, Number 2 ISSN 0736-1920 Summer 2015 OUR FUTURE: Projections of Work and Life Looking Ahead with the Journal,E\Steven E. Beasley, Managing Editor4 Introduction to this Issue, E\ Helen Harkness, PhD, Guest Editor5 Chapter 1: 7KH)XWXUHE\ Leigh Ellen Key8 Chapter 2: 7KH(QGRI:RUNDV:H.QRZ,Wby Andy Hines10 Chapter 3: -REVDQG&DUHHUVRQWKH)URQW/LQHRIWKH)XWXUHby Gary Marx20 Chapter 4: 6LOLFRQ9DOOH\DQGWKH1HZ5XOHVRI:RUNE\Gary A. Bolles28 Chapter 5: 7UDLQLQJ&KDOOHQJHV)DFLQJ(GXFDWLRQDQG7UDLQLQJ DQG&DUHHU'HYHORSPHQWLQWKH)XWXUHE\Timothy C. Mack40 Chapter 6: 2OGSHRSOHDUHSHRSOHWRRVROHW·VDFWDFFRUGLQJO\by Aubrey de Grey47 Chapter 7: &ULVLVRI+XPDQ&DSLWDOLQ$HURVSDFH,W·V$OO$ERXWWKH67(0 by Deborah Westphal51 Chapter 8: &ROOHJHIRU$OO²5HDOLW\RU)ODZHG0\WK"E\ -

Mixing Old and Young: Enhancing Rejuvenation and Accelerating Aging
Mixing old and young: enhancing rejuvenation and accelerating aging Ashley Lau, … , James L. Kirkland, Stefan G. Tullius J Clin Invest. 2019;129(1):4-11. https://doi.org/10.1172/JCI123946. Review Donor age and recipient age are factors that influence transplantation outcomes. Aside from age-associated differences in intrinsic graft function and alloimmune responses, the ability of young and old cells to exert either rejuvenating or aging effects extrinsically may also apply to the transplantation of hematopoietic stem cells or solid organ transplants. While the potential for rejuvenation mediated by the transfer of youthful cells is currently being explored for therapeutic applications, aspects that relate to accelerating aging are no less clinically significant. Those effects may be particularly relevant in transplantation with an age discrepancy between donor and recipient. Here, we review recent advances in understanding the mechanisms by which young and old cells modify their environments to promote rejuvenation- or aging-associated phenotypes. We discuss their relevance to clinical transplantation and highlight potential opportunities for therapeutic intervention. Find the latest version: https://jci.me/123946/pdf REVIEW The Journal of Clinical Investigation Mixing old and young: enhancing rejuvenation and accelerating aging Ashley Lau,1 Brian K. Kennedy,2,3,4,5 James L. Kirkland,6 and Stefan G. Tullius1 1Division of Transplant Surgery, Department of Surgery, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA. 2Departments of Biochemistry and Physiology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore. 3Singapore Institute for Clinical Sciences, Singapore. 4Agency for Science, Technology and Research (A*STAR), Singapore. -

Longevity Extension from a Socioeconomic Perspective: Plausibility, Misconceptions, and Potential Outcomes
Sound Decisions: An Undergraduate Bioethics Journal Volume 2 Issue 1 Article 4 2016-12-15 Longevity Extension from a Socioeconomic Perspective: Plausibility, Misconceptions, and Potential Outcomes Eric Ralph University of Puget Sound Follow this and additional works at: https://soundideas.pugetsound.edu/sounddecisions Part of the Bioethics and Medical Ethics Commons Recommended Citation Ralph, Eric (2016) "Longevity Extension from a Socioeconomic Perspective: Plausibility, Misconceptions, and Potential Outcomes," Sound Decisions: An Undergraduate Bioethics Journal: Vol. 2 : Iss. 1 , Article 4. Available at: https://soundideas.pugetsound.edu/sounddecisions/vol2/iss1/4 This Article is brought to you for free and open access by the Student Publications at Sound Ideas. It has been accepted for inclusion in Sound Decisions: An Undergraduate Bioethics Journal by an authorized editor of Sound Ideas. For more information, please contact [email protected]. Ralph: Longevity Extension Longevity Extension from a Socioeconomic Perspective: Plausibility, Misconceptions, and Potential Outcomes Eric Ralph Introduction In the last several decades, a significant amount of progress has been made in pursuits to better understand the process of aging and subsequently gain some level of control over it. Current theories of aging are admittedly lacking, but this has not prevented biogerontologists from drastically increasing the longevity of yeast, drosophilae, worms, and mice (Vaiserman, Moskalev, & Pasyukova 2015; Tosato, Zamboni et al. 2007; Riera & Dillin 2015). Wide-ranging successes with gene therapy and increased comprehension of the genetic components of aging have also recently culminated in numerous successes in extending the longevity of animals and the first human trial of a gene therapy to extend life through telomerase manipulation is already underway, albeit on a small scale (Mendell et al. -

"Anti-Aging Medicine" and "Successful Aging" Two Sides of the Same Coin? Views of Anti-Aging Practitioners
Flail. M.A., Seuerslen. R. A. Jr. , Ponsaran. R., & Fis hman. J.R. (20 1] ). Are ··:mt i-aging medicine'' and ··successful aging'' IWO sides of I he same coin ? Views of an ti-aging practitioners. Jmmwls or Geromolog y, Series 8: Psychological Scie11ces and Social Sciences. 68(6 ). 944--95 5. do i: 10.1093/geronb/gbt086. Ad va nce Access publicati on Septembe r 10. 20 13 Are "Anti-Aging Medicine" and "Successful Aging" Two Sides of the Same Coin? Views of Anti-Aging Practitioners Michael A. Flatt, 1 Richard A. Settersten Jr., 2 Roselle Ponsaran, 1 and Jennifer R. Fishman' 'Department of Bioethics, Case Western Reserve University, Cleveland, Ohio. 2Social and Behavioral Health Sciences, College of Public Health and Human Sciences, Oregon State University, Corvallis, Oregon. 3Biomedical Ethics Unit and the Department of the Social Studies of Medicine, McGill University, Quebec, Canada. Objectives. This article analyzes data from interviews with anti-aging practitioners to evaluate how their descriptions of the work they do, their definitions of aging, and their goals for their patients intersect with gerontological views of "successful aging." Method. Semistructured interviews were conducted with a sample of 3 I anti-aging practitioners drawn from the direc tory of the American Academy for Anti-Aging Medicine. Results. Qualitative analysis of the transcripts demonstrate that practitioners' descriptions of their goals, intentionall y or unintentionally, mimic the dominant models of "successful aging." These include lowered ri sk of disease and di sabil ity, maintenance of high levels of mental and physical function. and continuing social engagement. Yet, the means and modes of achieving·these goals differ markedly between the two groups, as do the messages that each group puts fo rth in defending their position s. -

Supercentenarians Landscape Overview
Supercentenarians Landscape Overview Top-100 Living Top-100 Longest-Lived Top-25 Socially and Professionally Active Executive and Infographic Summary GERONTOLOGY RESEARCH GROUP www.aginganalytics.com www.grg.org Supercentenarians Landscape Overview Foreword 3 Top-100 Living Supercentenarians Overview 44 Preface. How Long Can Humans Live and 4 Ages of Oldest Living Supercentenarians by Country 46 the Importance of Age Validation Top-100 Living Supercentenarians Continental Executive Summary 10 47 Distribution by Gender Introduction. 26 Top-100 Living Supercentenarians Distribution by Age 50 All Validated Supercentenarians Сhapter III. Top-25 Socially and Professionally Active All Supercentenarians Region Distribution by Gender 29 52 Living Centenarians Top-25 Socially and Professionally Active Centenarians All Supercentenarians Distribution by Nations 30 53 Overview Top-25 Socially and Professionally Active Centenarians Longest-Lived Supercentenarians Distribution by Country 31 54 Distribution by Nation Top-25 Socially and Professionally Active Centenarians All Supercentenarians Distribution by Gender and Age 32 55 Gender Distribution Top-25 Socially and Professionally Active Centenarians Сhapter I. Top-100 Longest-Lived Supercentenarians 35 56 Distribution by Type of Activity Chapter IV. Profiles of Top-100 Longest-Lived Top-100 Longest-Lived Supercentenarians Overview 36 57 Supercentenarians Top-100 Longest-Lived Supercentenarians Regional 38 Chapter V. Profiles of Top-100 Living Supercentenarians 158 Distribution by Gender Top-100 Longest-Lived Supercentenarians Distribution by Chapter VI. Profiles of Top-25 Socially and Professionally 40 259 Age Active Living Centenarians and Nonagenarians Сhapter II. Top-100 Living Supercentenarians 43 Disclaimer 285 Executive Summary There have always been human beings who have lived well beyond normal life expectancy, these ‘supercentenarians’ who lived past 110 years of age. -

From Here to Immortality: Anti-Aging Medicine
FromFrom HereHere toto Immortality:Immortaalitty: AAnti-AgingAnnntti-AAgging MMedicineedicine Anti-aging medicine is a $5 billion industry. Despite its critics, researchers are discovering that inter ventions designed to turn back time may prove to be more science than fiction. By Trudie Mitschang 14 BioSupply Trends Quarterly • October 2013 he symptoms are disturbing. Weight gain, muscle Shifting Attitudes Fuel a Booming Industry aches, fatigue and joint stiffness. Some experience The notion that aging requires treatment is based on a belief Thear ing loss and diminished eyesight. In time, both that becoming old is both undesirable and unattractive. In the memory and libido will lapse, while sagging skin and inconti - last several decades, aging has become synonymous with nence may also become problematic. It is a malady that begins dete rioration, while youth is increasingly revered and in one’s late 40 s, and currently 100 percent of baby boomers admired. Anti-aging medicine is a relatively new but thriving suffer from it. No one is immune and left untreated ; it always field driven by a baby- boomer generation fighting to preserve leads to death. A frightening new disease, virus or plague? No , its “forever young” façade. According to the market research it’s simply a fact of life , and it’s called aging. firm Global Industry Analysts, the boomer-fueled consumer The mythical fountain of youth has long been the subject of base will push the U.S. market for anti-aging products from folklore, and although it is both natural and inevitable, human about $80 billion now to more than $114 billion by 2015. -

SENS-Research-Foundation-2019
by the year 2050, cardiovascular an estimated 25-30 the american 85 percent of adults disease years and older age 85 or older remains the most population will suffer from common cause of 2 1 2 dementia. death in older adults. triple. THE CLOCK IS TICKING. By 2030, annual direct The estimated cost of medical costs associated dementia worldwide was 62% of Americans with cardiovascular $818 billion diseases in the united over age 65 have in 2015 and is states are expected to more than one expected to grow to rise to more than chronic condition.1 3 $2 trillion $818 billion. by 2030.1 References: (1) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5732407/, (2) https://www.who.int/ageing/publications/global_health.pdf, (3) https://www.cdcfoundation.org/pr/2015/heart-disease-and-stroke-cost-america-nearly-1-billion-day-medical-costs-lost-productivity sens research foundation board of directors Barbara Logan Kevin Perrott Bill Liao Chairperson Treasurer Secretary Michael Boocher Kevin Dewalt James O’Neill Jonathan Cain Michael Kope Frank Schuler 02 CONTENTS 2019 Annual Report 04 Letter From The CEO 06 Outreach & Fundraising 08 Finances 09 Donors erin ashford photography 14 Education 26 Investments 20 Conferences & Events 30 Research Advisory Board 23 Speaking Engagements 31 10 Years Of Research 24 Alliance 32 MitoSENS 34 LysoSENS 35 Extramural Research 38 Publications 39 Ways to Donate cover Photo (c) Mikhail Leonov - stock.adobe.com special 10th anniversary edition 03 FROM THE CEO It’s early 2009, and it’s very late at night. Aubrey, Jeff, Sarah, Kevin, and Mike are sitting around a large table covered in papers and half-empty food containers. -

World Population Ageing 2019
World Population Ageing 2019 Highlights ST/ESA/SER.A/430 Department of Economic and Social Affairs Population Division World Population Ageing 2019 Highlights United Nations New York, 2019 The Department of Economic and Social Affairs of the United Nations Secretariat is a vital interface between global policies in the economic, social and environmental spheres and national action. The Department works in three main interlinked areas: (i) it compiles, generates and analyses a wide range of economic, social and environmental data and information on which States Members of the United Nations draw to review common problems and take stock of policy options; (ii) it facilitates the negotiations of Member States in many intergovernmental bodies on joint courses of action to address ongoing or emerging global challenges; and (iii) it advises interested Governments on the ways and means of translating policy frameworks developed in United Nations conferences and summits into programmes at the country level and, through technical assistance, helps build national capacities. The Population Division of the Department of Economic and Social Affairs provides the international community with timely and accessible population data and analysis of population trends and development outcomes for all countries and areas of the world. To this end, the Division undertakes regular studies of population size and characteristics and of all three components of population change (fertility, mortality and migration). Founded in 1946, the Population Division provides substantive support on population and development issues to the United Nations General Assembly, the Economic and Social Council and the Commission on Population and Development. It also leads or participates in various interagency coordination mechanisms of the United Nations system. -

Shared Ageing Research Models (Sharm): a New Facility to Support Ageing Research
Biogerontology (2013) 14:789–794 DOI 10.1007/s10522-013-9457-0 METHOD Shared Ageing Research Models (ShARM): a new facility to support ageing research Adele L. Duran • Paul Potter • Sara Wells • Tom Kirkwood • Thomas von Zglinicki • Anne McArdle • Cheryl Scudamore • Qing-Jun Meng • Gerald de Haan • Anne Corcoran • Ilaria Bellantuono Received: 5 July 2013 / Accepted: 16 August 2013 / Published online: 2 October 2013 Ó The Author(s) 2013. This article is published with open access at Springerlink.com Abstract In order to manage the rise in life expec- Wellcome Trust, open to all investigators. It collects, tancy and the concomitant increased occurrence of stores and distributes flash frozen tissues from aged age-related diseases, research into ageing has become murine models through its biorepository and provides a strategic priority. Mouse models are commonly a database of live ageing mouse colonies available in utilised as they share high homology with humans and the UK and abroad. It also has an online environment show many similar signs and diseases of ageing. (MICEspace) for collation and analysis of data from However, the time and cost needed to rear aged communal models and discussion boards on subjects cohorts can limit research opportunities. Sharing of such as the welfare of ageing animals and common resources can provide an ethically and economically endpoints for intervention studies. Since launching in superior framework to overcome some of these issues July 2012, thanks to the generosity of researchers in but requires dedicated infrastructure. Shared Ageing UK and Europe, ShARM has collected more than Research Models (ShARM) (www.ShARMUK.org) 2,500 tissues and has in excess of 2,000 mice regis- is a new, not-for-profit organisation funded by tered in live ageing colonies. -
Viewer Comments
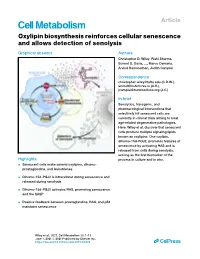
Article Oxylipin biosynthesis reinforces cellular senescence and allows detection of senolysis Graphical abstract Authors Christopher D. Wiley, Rishi Sharma, Sonnet S. Davis, ..., Marco Demaria, Arvind Ramanathan, Judith Campisi Correspondence [email protected] (C.D.W.), [email protected] (A.R.), [email protected] (J.C.) In brief Senolytics, transgenic, and pharmacological interventions that selectively kill senescent cells are currently in clinical trials aiming to treat age-related degenerative pathologies. Here, Wiley et al. discover that senescent cells produce multiple signaling lipids known as oxylipins. One oxylipin, dihomo-15d-PGJ2, promotes features of senescence by activating RAS and is released from cells during senolysis, serving as the first biomarker of the Highlights process in culture and in vivo. d Senescent cells make several oxylipins, dihomo- prostaglandins, and leukotrienes d Dihomo-15d-PGJ2 is intracellular during senescence and released during senolysis d Dihomo-15d-PGJ2 activates RAS, promoting senescence and the SASP d Positive feedback between prostaglandins, RAS, and p53 maintains senescence Wiley et al., 2021, Cell Metabolism 33, 1–13 June 1, 2021 ª 2021 Published by Elsevier Inc. https://doi.org/10.1016/j.cmet.2021.03.008 ll Please cite this article in press as: Wiley et al., Oxylipin biosynthesis reinforces cellular senescence and allows detection of senolysis, Cell Metabolism (2021), https://doi.org/10.1016/j.cmet.2021.03.008 ll Article Oxylipin biosynthesis reinforces cellular senescence and allows detection of senolysis Christopher D. Wiley,1,2,* Rishi Sharma,1 Sonnet S. Davis,1 Jose Alberto Lopez-Dominguez,1 Kylie P. Mitchell,1 Samantha Wiley,1 Fatouma Alimirah,1 Dong Eun Kim,1 Therese Payne,1 Andrew Rosko,1 Eliezer Aimontche,1 Sharvari M. -

Read Our New Annual Report
The seeds of a concept. The roots of an idea. The potential of a world free of age-related disease. Photo: Sherry Loeser Photography SENS Research Foundation Board of Directors Barbara Logan, Chair Bill Liao, Secretary Kevin Perrott, Treasurer Michael Boocher Jonathan Cain Kevin Dewalt Michael Kope Jim O’Neill Frank Schüler Sherry Loeser Photography 2 Contents CEO Letter (Jim O’Neill) 4 Finances 5 Donors 6 - 7 Fundraising & Conferences 8 - 9 Around the World with Aubrey de Grey 10 Outreach 11 Founding CEO Tribute & Underdog Pharmaceuticals 12 - 13 Investments 14 Welcome New Team Members 15 Education 16 - 17 Publications & Research Advisory Board 18 Research Summaries 19 - 22 Ways to Donate 23 The SRF Team Front row: Anne Corwin (Engineer/Editor), Amutha Boominathan (MitoSENS Group Lead), Alexandra Stolzing (VP of Research), Aubrey de Grey (Chief Science Officer), Jim O’Neill (CEO), Bhavna Dixit (Research Associate). Center row: Caitlin Lewis (Research Associate), Lisa Fabiny-Kiser (VP of Operations), Gary Abramson (Graphics), Maria Entraigues-Abramson (Global Outreach Coordinator), Jessica Lubke (Administrative Assistant). Back row: Tesfahun Dessale Admasu (Research Fellow), Amit Sharma (ImmunoSENS Group Lead), Michael Rae (Science Writer), Kelly Protzman (Executive Assistant). Not Pictured: Greg Chin (Director, SRF Education), Ben Zealley (Website/Research Assistant/ Deputy Editor) Photo: Sherry Loeser Photography, 2019 3 From the CEO At our 2013 conference at Queens College, Cambridge, I closed my talk by saying, “We should not rest until we make aging an absurdity.” We are now in a very different place. After a lot of patient explanation, publication of scientific results, conferences, and time, our community persuaded enough scientists of the feasibility of the damage repair approach to move SENS and SENS Research Foundation from the fringes of scientific respectability to the vanguard of a mainstream community of scientists developing medical therapies to tackle human aging.