Flamingo, Special Publication 1, January 2009
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ardea Cinerea (Grey Heron) Family: Ardeidae (Herons and Egrets) Order: Ciconiiformes (Storks, Herons and Ibises) Class: Aves (Birds)
UWU The Online Guide to the Animals of Trinidad and Tobago Behaviour Ardea cinerea (Grey Heron) Family: Ardeidae (Herons and Egrets) Order: Ciconiiformes (Storks, Herons and Ibises) Class: Aves (Birds) Fig. 1. Grey heron, Ardea cinerea. [http://www.google.tt/imgres?imgurl=http://www.bbc.co.uk/lancashire/content/images/2006/06/15/grey_heron, downloaded 14 November 2012] TRAITS. Grey herons are large birds that can be 90-100cm tall and an adult could weigh in at approximately 1.5 kg. They are identified by their long necks and very powerful dagger like bills (Briffett 1992). They have grey plumage with long black head plumes and their neck is white with black stripes on the front. In adults the forehead sides of the head and the centre of the crown are white. In flight the neck is folded back with the wings bowed and the flight feathers are black. Each gender looks alike except for the fact that females have shorter heads (Seng and Gardner 1997). The juvenile is greyer without black markings on the head and breast. They usually live long with a life span of 15-24 years. ECOLOGY. The grey heron is found in Europe, Asia and Africa, and has been recorded as an accidental visitor in Trinidad. Grey herons occur in many different habitat types including savannas, ponds, rivers, streams, lakes and temporary pools, coastal brackish water, wetlands, marsh and swamps. Their distribution may depend on the availability of shallow water (brackish, saline, fresh, flowing and standing) (Briffett 1992). They prefer areas with tall trees for nesting UWU The Online Guide to the Animals of Trinidad and Tobago Behaviour (arboreal rooster and nester) but if trees are unavailable, grey herons may roost in dense brush or undergrowth. -

Catalan Modernism and Vexillology
Catalan Modernism and Vexillology Sebastià Herreros i Agüí Abstract Modernism (Modern Style, Modernisme, or Art Nouveau) was an artistic and cultural movement which flourished in Europe roughly between 1880 and 1915. In Catalonia, because this era coincided with movements for autonomy and independence and the growth of a rich bourgeoisie, Modernism developed in a special way. Differing from the form in other countries, in Catalonia works in the Modern Style included many symbolic elements reflecting the Catalan nationalism of their creators. This paper, which follows Wladyslaw Serwatowski’s 20 ICV presentation on Antoni Gaudí as a vexillographer, studies other Modernist artists and their flag-related works. Lluís Domènech i Montaner, Josep Puig i Cadafalch, Josep Llimona, Miquel Blay, Alexandre de Riquer, Apel·les Mestres, Antoni Maria Gallissà, Joan Maragall, Josep Maria Jujol, Lluís Masriera, Lluís Millet, and others were masters in many artistic disciplines: Architecture, Sculpture, Jewelry, Poetry, Music, Sigillography, Bookplates, etc. and also, perhaps unconsciously, Vexillography. This paper highlights several flags and banners of unusual quality and national significance: Unió Catalanista, Sant Lluc, CADCI, Catalans d’Amèrica, Ripoll, Orfeó Català, Esbart Català de Dansaires, and some gonfalons and flags from choral groups and sometent (armed civil groups). New Banner, Basilica of the Monastery of Santa Maria de Ripoll Proceedings of the 24th International Congress of Vexillology, Washington, D.C., USA 1–5 August 2011 © 2011 North American Vexillological Association (www.nava.org) 506 Catalan Modernism and Vexillology Background At the 20th International Conference of Vexillology in Stockholm in 2003, Wladyslaw Serwatowski presented the paper “Was Antonio Gaudí i Cornet (1852–1936) a Vexillographer?” in which he analyzed the vexillological works of the Catalan architectural genius Gaudí. -

IUCN-SSC Stork, Ibis and Spoonbill Specialist Group Special Publication 2
IUCN-SSC Stork, Ibis and Spoonbill Specialist Group Special Publication 2 Proceedings of the IX Workshop of the AEWA Eurasian Spoonbill International Expert Group Djerba Island, Tunisia, 14th - 18th November 2018 Editors: Jocelyn Champagnon, Jelena Kralj, Luis Santiago Cano Alonso and K. S. Gopi Sundar Editors-in-Chief, Special Publications, IUCN-SSC Stork, Ibis and Spoonbill Specialist Group K.S. Gopi Sundar, Co-chair IUCN Stork, Ibis and Spoonbill Specialist Group Luis Santiago Cano Alonso, Co-chair IUCN Stork, Ibis and Spoonbill Specialist Group Invited Editors for this issue Jocelyn Champagnon, Tour du Valat, Research Institute for the Conservation of Mediterranean Wetlands, Arles, France Jelena Kralj, Institute of Ornithology, Zagreb, Croatia Expert Review Board Hichem Azafzaf, Association “les Amis des Oiseaux » (AAO/BirdLife Tunisia), Tunisia Petra de Goeij, Royal NIOZ, the Netherlands Csaba Pigniczki, Kiskunság National Park Directorate, Hungary Suggested citation of this publication: Champagnon J., Kralj J., Cano Alonso, L. S. & Sundar, K. S. G. (ed.) 2019. Proceedings of the IX Workshop of the AEWA Eurasian Spoonbill International Expert Group. IUCN-SSC Stork, Ibis and Spoonbill Specialist Group Special Publication 2. Arles, France. ISBN 978-2-491451-00-4. Recommended Citation of a chapter: Marion L. 2019. Recent trends of the breeding population of Spoonbill in France 2012- 2018. Pp 19- 23. In: Champagnon J., Kralj J., Cano Alonso, L. S. & Sundar, K. S. G. (ed.) Proceedings of the IX Workshop of the AEWA Eurasian Spoonbill International Expert Group. IUCN-SSC Stork, Ibis and Spoonbill Specialist Group Special Publication 2. Arles, France. INFORMATION AND WRITING DISCLAIMER The information and opinions expressed in this publication belong to the authors. -

Flamingo ABOUT the GROUP
Flamingo ABOUT THE GROUP Bulletin of the IUCN-SSC/Wetlands International The Flamingo Specialist Group (FSG) was established in 1978 at Tour du Valat in France, under the leadership of Dr. Alan Johnson, who coordinated the group until 2004 (see profile at www.wetlands.org/networks/Profiles/January.htm). Currently, the group is FLAMINGO SPECIALIST GROUP coordinated from the Wildfowl & Wetlands Trust at Slimbridge, UK, as part of the IUCN- SSC/Wetlands International Waterbird Network. The FSG is a global network of flamingo specialists (both scientists and non- scientists) concerned with the study, monitoring, management and conservation of the world’s six flamingo species populations. Its role is to actively promote flamingo research and conservation worldwide by encouraging information exchange and cooperation amongst these specialists, and with other relevant organisations, particularly IUCN - SSC, Ramsar, WWF International and BirdLife International. FSG members include experts in both in-situ (wild) and ex-situ (captive) flamingo conservation, as well as in fields ranging from field surveys to breeding biology, diseases, tracking movements and data management. There are currently 165 members around the world, from India to Chile, and from France to South Africa. Further information about the FSG, its membership, the membership list serve, or this bulletin can be obtained from Brooks Childress at the address below. Chair Assistant Chair Dr. Brooks Childress Mr. Nigel Jarrett Wildfowl & Wetlands Trust Wildfowl & Wetlands Trust Slimbridge Slimbridge Glos. GL2 7BT, UK Glos. GL2 7BT, UK Tel: +44 (0)1453 860437 Tel: +44 (0)1453 891177 Fax: +44 (0)1453 860437 Fax: +44 (0)1453 890827 [email protected] [email protected] Eastern Hemisphere Chair Western Hemisphere Chair Dr. -

Wetland Birds in the Recent Fossil Record of Britain and Northwest Europe John R
Wetland birds in the recent fossil record of Britain and northwest Europe John R. Stewart 18. Dalmatian Pelican Pelecanus crispus, Deep Bay, Mai Po, Hong Kong, February 1995. Geological evidence suggests that Dalmatian Pelicans bred in Britain, and in other western European countries (including The Netherlands and Denmark), prior to and during the Iron Age. Ray Tipper. ABSTRACT Wetland habitats in Britain and other parts of western Europe have been severely depleted during the latter part of the Holocene owing principally to drainage and land reclamation. Changes in the distribution of a number of wetland bird species can be gauged from archaeological and geological site records of larger birds, whose remains are generally better preserved. Key species are discussed here, including a heron Nycticorax fenensis and a crane Grus primigenia, two extinct species named on possibly uncertain fossil evidence. We can let our minds wander back to the misty realms of fifteen hundred years ago, to a wonderful Britain which was alive with bird song from coast to coast, which sheltered wolves, bears and boars in its dark woodlands, cranes in its marshes, bustards on its heaths and beavers by its streams, and we can visualize the great pink pelican sweeping on its huge pinions over the reedy waterways which then penetrated by secret paths into the very heart of what is now Somerset. (Whitlock, 1953) © British Birds 97 • January 2004 • 33-43 33 Wetland birds in the recent fossil record f all the major habitats in northwest species, including Mute Swan Cygnus olor and Europe, wetlands may have been the Common Crane, may have become physically Omost severely depleted during the smaller owing to habitat impoverishment. -

Scaling up for Impact: Europe Regional Strategy 2015 - 2025
Scaling up for impact: Europe regional strategy 2015 - 2025 Scaling up for impact: Europe Regional strategy 2015 – 2025 Wetlands International September 2016 Cover photo: Sava river. Photo by Romy Durst. 2 Wetlands International European Regional Strategy 2015 - 2025 Contents Introduction ............................................................................................................................................ 3 Our vision and mission ............................................................................................................................ 4 Our approach .......................................................................................................................................... 4 Why our work is needed ......................................................................................................................... 5 Our strategy ............................................................................................................................................ 6 How we work in Europe .................................................................................................................. 7 Geographical focus.......................................................................................................................... 8 Our niche and added value ............................................................................................................. 9 Our target groups ......................................................................................................................... -
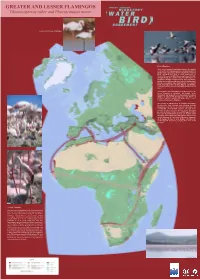
GREATER and LESSER FLAMINGOS Phoenicopterus Ruber and Phoeniconaias Minor
GREATER AND LESSER FLAMINGOS Phoenicopterus ruber and Phoeniconaias minor Greater and Lesser Flamingos © Cliff Buckton © P & H Harris Lesser Flamingo The Lesser Flamingo Phoeniconaias minor is the smallest of the world's five flamingo species. It occurs primarily in the Rift Valley lakes of East Africa with about 4 to 5 million birds estimated, but also in small populations in Namibia/Botswana (40,000), Mauritania/Senegal (15,400), Ethiopia (8,300). The alkaline lakes of the Rift Valley are the primary feeding areas for the East Africa population. During non-breeding periods these lakes often hold almost the entire population. Huge feeding flocks of 1-2 million birds frequently gather on lakes Bogoria and Nakuru, creating one of the most stunning wildlife spectacles in the world. Although it is still the most numerous of the five species, the Lesser Flamingo is classified as globally "near threatened" due primarily to its dependence on a limited number of unprotected breeding sites and threats of proposed soda-ash mining and hydro-electric power schemes on the main breeding lakes. The question of whether there is occasional interchange between the East African and southern African populations has yet to be resolved definitely, but considerable circumstantial evidence has now been assembled to show that East African Lesser Flamingos probably do fly to Botswana to breed during periods when the Lake Makgadikgadi Salt Pans are flooded. Their migration routes, flight range and stopover places (if any) are still unknown. It is now known that Lesser Flamingos do fly during the day, at great heights, well above the normal diurnal movement of eagles, their main aerial predator. -

Flamingo Newsletter 17, 2009
ABOUT THE GROUP The Flamingo Specialist Group (FSG) is a global network of flamingo specialists (both scientists and non-scientists) concerned with the study, monitoring, management and conservation of the world’s six flamingo species populations. Its role is to actively promote flamingo research, conservation and education worldwide by encouraging information exchange and cooperation among these specialists, and with other relevant organisations, particularly the IUCN Species Survival Commission (SSC), the Ramsar Convention on Wetlands, the Convention on Conservation of Migratory Species (CMS), the African-Eurasian Migratory Waterbird Agreement (AEWA), and BirdLife International. The group is coordinated from the Wildfowl & Wetlands Trust, Slimbridge, UK, as part of the IUCN-SSC/Wetlands International Waterbird Network. FSG members include experts in both in-situ (wild) and ex-situ (captive) flamingo conservation, as well as in fields ranging from research surveys to breeding biology, infectious diseases, toxicology, movement tracking and data management. There are currently 286 members representing 206 organisations around the world, from India to Chile, and from France to South Africa. Further information about the FSG, its membership, the membership list serve, or this bulletin can be obtained from Brooks Childress at the address below. Chair Dr. Brooks Childress Wildfowl & Wetlands Trust Slimbridge Glos. GL2 7BT, UK Tel: +44 (0)1453 860437 Fax: +44 (0)1453 860437 [email protected] Eastern Hemisphere Chair Western Hemisphere Chair Dr. Arnaud Béchet Dr. Felicity Arengo Station biologique, Tour du Valat American Museum of Natural History Le Sambuc Central Park West at 79th Street 13200 Arles, France New York, NY 10024 USA Tel : +33 (0) 4 90 97 20 13 Tel: +1 212 313-7076 Fax : +33 (0) 4 90 97 20 19 Fax: +1 212 769-5292 [email protected] [email protected] Citation: Childress, B., Arengo, F. -

Resource-Dependent Weather Effect in the Reproduction of the White Stork Ciconia Ciconia
Resource-dependent weather effect in the reproduction of the White Stork Ciconia ciconia Damijan Denac1 Denac D. 2006. Resource-dependent weather effect in the reproduction of the White Stork Ciconia ciconia. Ardea 94(2): 233–240. Weather affects the breeding success of White Stork Ciconia ciconia, but the effect has not been studied in the context of different food resources or habitat quality. The aim of this study was to determine whether the impact of weather conditions on breeding success was dependent on habitat quality. The effect of weather on reproduction was analysed in two populations that differed significantly in the availability of suitable feeding habitats. Multiple regression analyses revealed that of the weather variables analysed (average temperature and rainfall in April, May and June), rainfall in May and temperature in June explained a sig- nificant part of the variation in numbers of fledged chicks per pair, but only in the population with poorer food resources. The lack of weather influence in the population with richer food resources was tentatively explained by the larger brood sizes. More effective heat conservation in larger broods, and thus lower chick mortality during cold weather, could be the underlying mechanism for the different response to weather in the two populations. Key words: breeding success, Ciconia ciconia, habitat quality, resource dependent weather-effect, White Stork 1National Institute of Biology, Vecv na pot 111, SI–1000 Ljubljana, Slovenia ([email protected]) INTRODUCTION optimal feeding habitats, whereas fields, especially cornfields, are suboptimal (Sackl 1987, Pinowski et The influences of food as a resource and weather al. -

A Year Following Egrets
❚ 10 The Ardeid ❚■ Insights from birds with GPS tags A Year Following Egrets by David Lumpkin o a Great Egret (Ardea alba), Tomales Bay the deployment of our tags, all three Tis full of food, but that food is not always egrets remained near Toms Point in available. Every two weeks, around the full northern Tomales Bay, where we and new moons, the lowest tides and greatest could regularly download data foraging opportunity coincide with the early with the handheld receiver Figure 1. Egret 9 flying over Cypress morning, making breakfast on the bay an easy then eagerly rush back Grove the day after it was captured in affair. During low tides, hundreds of acres of to the office to upload September 2018. intertidal eelgrass are exposed, allowing egrets the data to a computer to stab at herring during spawning events or and see what the birds had been doing. About a to hunt pipefish, which try to wrap themselves month after tagging, suddenly two of the three a freshwater pond at Toms Point. I watched around the egret’s bill to avoid being swal- egrets could no longer be found on Tomales him forage there several times. Though this lowed. As the tide cycle shifts and morning tides Bay. Worried something might have happened pond was well covered by aquatic plants, he was become higher, the eelgrass is exposed for fewer to them, and perplexed that they might leave an consistently able to find small fish, picking them hours per day, reducing foraging opportunities area where they likely had active nests, I drove out from tiny gaps in foliage. -

Mediterranean Gulls Larus Melanocephalus Wintering Along the Mediterranean Iberian Coast: Numbers and Activity Rhythms in the Species’ Main Winter Quarters
J Ornithol DOI 10.1007/s10336-011-0673-6 ORIGINAL ARTICLE Mediterranean Gulls Larus melanocephalus wintering along the Mediterranean Iberian coast: numbers and activity rhythms in the species’ main winter quarters Albert Cama • Pere Josa • Joan Ferrer-Obiol • Jose´ Manuel Arcos Received: 22 June 2010 / Revised: 23 December 2010 / Accepted: 14 February 2011 Ó Dt. Ornithologen-Gesellschaft e.V. 2011 Abstract Knowledge of the winter distribution of the which recent data are lacking. Information for the whole Mediterranean Gull Larus melanocephalus is poor. The study area was obtained from a systematic boat-based limited and geographically patchy data on the species’ survey over the continental shelf in 2003. Then, particular winter distribution is of concern because current estimates attention was paid to St. Jordi Gulf, a known hotspot for of the wintering population do not agree with those for the the species, where we studied the temporal patterns in global breeding population. We assessed the winter dis- abundance throughout the winter months, and daily tribution and abundance patterns of this gull in Mediter- activity rhythms, between 2005–2006 and 2008–2009. To ranean Iberia, a historically important wintering area for set our results in a global context, the available informa- tion of the winter range of the species was collated and synthesised. The results indicate that the Iberian Medi- terranean coast is the main winter quarters of the species. An average population of ca. 41,000 individuals was Communicated by P. H. Becker. present in the area, representing approximately half the Electronic supplementary material The online version of this 86,311 individuals (range 50,747–121,875) recorded article (doi:10.1007/s10336-011-0673-6) contains supplementary across the whole of the species’ winter range. -

PRESS RELEASE Royal Boskalis Westminster N.V
PRESS RELEASE Royal Boskalis Westminster N.V. Boskalis and Wetlands International to explore the PO Box 43 3350 AA Papendrecht potential of blue carbon The Netherlands Papendrecht, 3 February 2020 Page 1 | 2 Royal Boskalis Westminster N.V. (Boskalis), a global dredging and marine contractor and Wetlands International, the global NGO dedicated to the safeguarding and restoring of wetlands, will intensify collaboration to enhance and restore coastal wetland habitats that not only support coastal protection and fisheries but store some of the world’s largest quantities of carbon. The recently signed agreement will see the organizations first focus on developing the expertise and knowledge of ‘blue carbon’ ecosystems that can contribute to climate targets, adaptation and biodiversity conservation. ‘Blue carbon’ refers to the carbon stored by the world's coastal ecosystems, mostly mangroves, salt marshes and seagrasses. With increasing risks of flooding and erosion, the impacts of climate change on coastal systems and communities are becoming ever more apparent. The dredging sector has a large role to play in safeguarding these areas, providing innovative ways to protect coastlines and introducing adaptive measures. Jane Madgwick, Chief Executive Officer of Wetlands International said: “As natural coastal protection, carbon and water stores, wetlands are vital in helping communities and nature deal with the effects of climate change. Together with Boskalis, we aim to further develop our blue carbon knowledge and expertise to help nature-based approaches become best practice across the maritime and dredging sector.” Theo Baartmans, Board Member and Chief Operating Officer, Boskalis: “As a leader in the industry, Boskalis is keen to further develop nature-based solutions to protect and enrich coastal ecosystems from the consequences of climate change.