Abstract Book
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Emissions of Such Gases on a ‘CO2- Equivalent’ Scale
Department of Meteorology Assessing the potential climate impacts of industrial gases Professor Keith Shine FRS | Professor Eleanor Highwood Summary Human activity leads to the emission of many greenhouse gases that differ from carbon dioxide (CO2) in their effects on climate. International climate policy requires the use of an ‘exchange rate’ to place emissions of such gases on a ‘CO2- equivalent’ scale. These ‘exchange rates’ are calculated using ‘climate emission metrics’, which enable quantitative comparisons to be made of the climate impact of the emission of a given gas with respect to CO2 emissions. Background The assessment reports of the Intergovernmental Panel on Climate Change (IPCC) and the World Meteorological Organization / United Nations Environment Programme (WMO/UNEP) Scientific Assessments of Stratospheric Ozone Depletion presented the values of ‘Global Warming Potential’ or GWP. GWP is the metric adopted by the Kyoto Protocol to the United Nations’ Framework Convention on Climate Change (UNFCCC) to allow signatories to report emissions of different greenhouse gases on a CO2-equivalent scale, and is one of a range of possible methods for comparing the climate impact of emissions of different greenhouse gases. How is University of Reading research contributing? Professor Keith Shine played a major role in the international assessments. He led the compilation of databases of an essential input to GWP calculations, namely the so-called ‘radiative efficiency’, or RE, for gases included in the Kyoto Protocol. He and his co-workers within and outside the Department of Meteorology developed and refined methods for calculating RE, using advanced numerical models incorporating new laboratory observations. For many industrial gases, the Reading group has presented the first published RE value, and it has helped resolve instances where results presented in the literature had been in substantive disagreement. -

Abstracts Connecting to the Boston University Network
20th Cambridge Workshop: Cool Stars, Stellar Systems, and the Sun July 29 - Aug 3, 2018 Boston / Cambridge, USA Abstracts Connecting to the Boston University Network 1. Select network ”BU Guest (unencrypted)” 2. Once connected, open a web browser and try to navigate to a website. You should be redirected to https://safeconnect.bu.edu:9443 for registration. If the page does not automatically redirect, go to bu.edu to be brought to the login page. 3. Enter the login information: Guest Username: CoolStars20 Password: CoolStars20 Click to accept the conditions then log in. ii Foreword Our story starts on January 31, 1980 when a small group of about 50 astronomers came to- gether, organized by Andrea Dupree, to discuss the results from the new high-energy satel- lites IUE and Einstein. Called “Cool Stars, Stellar Systems, and the Sun,” the meeting empha- sized the solar stellar connection and focused discussion on “several topics … in which the similarity is manifest: the structures of chromospheres and coronae, stellar activity, and the phenomena of mass loss,” according to the preface of the resulting, “Special Report of the Smithsonian Astrophysical Observatory.” We could easily have chosen the same topics for this meeting. Over the summer of 1980, the group met again in Bonas, France and then back in Cambridge in 1981. Nearly 40 years on, I am comfortable saying these workshops have evolved to be the premier conference series for cool star research. Cool Stars has been held largely biennially, alternating between North America and Europe. Over that time, the field of stellar astro- physics has been upended several times, first by results from Hubble, then ROSAT, then Keck and other large aperture ground-based adaptive optics telescopes. -

Metrics for Comparison of Climate Impacts from Well Mixed
1 ACCRI Theme 7 2 3 Metrics for comparison of climate impacts from well mixed greenhouse gases and 4 inhomogeneous forcing such as those from UT/LS ozone, contrails and contrail- 5 cirrus 6 7 Piers Forster & Helen Rogers 8 9 Acknowledgement: The numbers in Table 5 and a many of the ideas are derived from a 10 unpublished manuscript led by Keith Shine that Piers Forster and Helen Rogers were co- 11 author of. 12 13 Executive Summary.......................................................................................................... 2 14 1. Introduction and Background ................................................................................. 5 15 2. Review ........................................................................................................................ 8 16 2.1. Current state of science....................................................................................... 8 17 2.1.1. Air travel – its emissions and its trends ...................................................... 8 18 2.1.2. Aviation’s climate impact......................................................................... 10 19 2.1.3. Review of the RF characteristics and uncertainties of mechanisms ......... 12 20 2.1.3.1. Chemistry of importance to aviation..................................................... 12 21 2.1.3.2. Modelling the impact of aviation.......................................................... 14 22 2.1.4. Regional and timescale issues................................................................... 16 23 2.2. Critical -

Trends and Patterns in the Contributions to Cumulative Radiative Forcing from Different Regions of the World
Trends and patterns in the contributions to cumulative radiative forcing from different regions of the world D. M. Murphya,1 and A. R. Ravishankarab,c,1 aEarth System Research Laboratory, Chemical Sciences Division, National Oceanic and Atmospheric Administration, Boulder, CO 80305; bDepartment of Chemistry, Colorado State University, Fort Collins, CO 80524; and cDepartment of Atmospheric Science, Colorado State University, Fort Collins, CO 80524 Contributed by A. R. Ravishankara, November 12, 2018 (sent for review August 17, 2018; reviewed by John H. Seinfeld and Keith Shine) Different regions of the world have had different historical very short lived, on the order of weeks, so that their radiative patterns of emissions of carbon dioxide, other greenhouse gases, influence is essentially simultaneous with their emission time. and aerosols as well as different land-use changes. One can estimate The relative emissions of GHGs and aerosols have not been the net cumulative contribution by each region to the global mean the same in different regions. One reason is that some regions radiative forcing due to past greenhouse gas emissions, aerosol have had more emissions from industrial activity than other re- precursors, and carbon dioxide from land-use changes. Several gions, and in other regions land-use/land-cover change (LULC) patterns stand out from such calculations. Some regions have had a has been an important driver of emissions. Here we explicitly common historical pattern in which the short-term offsets between deal with only the largest component of the latter––the influence the radiative forcings from carbon dioxide and sulfate aerosols of LULC on CO2. -

Fall/Winter 2003 Print Issue
T he Magazine of San 360Diego State University F all/Winter 2003 Welcome to 360 online! To increase the type size for easier reading, change the percentage field in your toolbar or use the settings found under the “view” tab. To jump from one article to another, use the “table of contents” or “thumbnail” links under the tabs to the left. If no tabs appear, click on the navigation symbol in your toolbar to reveal them. International Inspiration. SDSU students are taking flight as citizens of the world. Real-World Referee. FTC chair Tim Muris rules for consumers and fair competition. One Singular Sensation. Musical theatre hopefuls polish their acts. Teaching Teachers. San Diego State’s original mandate remains a top priority. The English word “excellence” comes from the Latin “excellere,” meaning “to climb higher.” Excellence is not about elitism; it is about life’s We invite you to join us in our climb. SDSU is elemental core: the struggle to fully express and increasingly recognized among the nation’s major expand one’s capabilities. urban universities. With more than 39,000 appli- cants competing for fewer than 7,300 undergraduate California’s fiscal and political difficulties do not vacancies this fall, our incoming freshmen are better exempt San Diego State from its responsibilities for prepared than ever before. Their average GPA is excellence. We will continue our important work – estimated at 3.5; their average SAT is projected at providing a high-quality learning experience for our 1071. We expect great things of these newest Aztecs students, supporting our faculty in their teaching as they pursue their education and then move on to and research, and serving our community as a assume positions of responsibility and leadership in resource and problem-solver. -

Chapter 11 CORINTHIAN COLLEGES, INC., Et Al. Case
Case 15-10952-KJC Doc 712 Filed 08/05/15 Page 1 of 2014 IN THE UNITED STATES BANKRUPTCY COURT FOR THE DISTRICT OF DELAWARE In re: Chapter 11 CORINTHIAN COLLEGES, INC., et al.1 Case No. 15-10952-CSS Debtor. AFFIDAVIT OF SERVICE STATE OF CALIFORNIA } } ss.: COUNTY OF LOS ANGELES } SCOTT M. EWING, being duly sworn, deposes and says: 1. I am employed by Rust Consulting/Omni Bankruptcy, located at 5955 DeSoto Avenue, Suite 100, Woodland Hills, CA 91367. I am over the age of eighteen years and am not a party to the above-captioned action. 2. On July 30, 2015, I caused to be served the: a) Notice of (I) Deadline for Casting Votes to Accept or Reject the Debtors’ Plan of Liquidation, (II) The Hearing to Consider Confirmation of the Combined Plan and Disclosure Statement and (III) Certain Related Matters, (the “Confirmation Hearing Notice”), b) Debtors’ Second Amended and Modified Combined Disclosure Statement and Chapter 11 Plan of Liquidation, (the “Combined Disclosure Statement/Plan”), c) Class 1 Ballot for Accepting or Rejecting Debtors’ Chapter 11 Plan of Liquidation, (the “Class 1 Ballot”), d) Class 4 Ballot for Accepting or Rejecting Debtors’ Chapter 11 Plan of Liquidation, (the “Class 4 Ballot”), e) Class 5 Ballot for Accepting or Rejecting Debtors’ Chapter 11 Plan of Liquidation, (the “Class 5 Ballot”), f) Class 4 Letter from Brown Rudnick LLP, (the “Class 4 Letter”), ____________________________________________________________________________________________________________________________________________________________________________________________________________ 1 The Debtors in these cases, along with the last four digits of each Debtor’s federal tax identification number, are: Corinthian Colleges, Inc. -
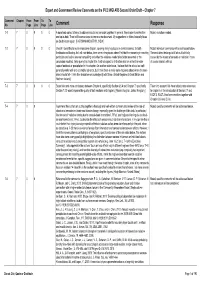
Comment Response 7-1 7 0 0 0 0 a Novel and Correct Attempt to Address Clouds and Aerosols Together
Expert and Government Review Comments on the IPCC WGI AR5 Second Order Draft – Chapter 7 Comment Chapter From From To To No Page Line Page Line Comment Response 7-1 7 0 0 0 0 A novel and correct attempt to address clouds and aerosols together. In general, the chapter is well-written Noted, no action needed. and up-to-date. There still is some scope to improve the document. My suggestions to follow, basically focus on South Asian region [K KRISHNA MOORTHY, INDIA] 7-2 7 0 0 0 0 Overall I found this to be an impressive chapter, covering many key topics in climate science, for both Noted. Individual comments will be addressed below. feedbacks and forcing. As I will note below, there were a few places where I felt that the reasoning in reaching The semi-direct belongs to AFari as it is initially particular conclusions was not compelling and either the evidence needs to be better presented or the caused by the impact of aerosols on radiation. It can conclusion modified. I also query the chapter title. I did not expect to find information on either the water of course interact with aci. vapour feedback or precipitation in this chapter. On another wider issue, I believe that the ari and aci split generally works well and is a helpful advance, but I think there is really some haziness about where the semi- direct should fall - I think this should be acknowledged [Keith Shine, United Kingdom of Great Britain and Northern Ireland] 7-3 7 0 0 0 0 Better links and more consistency between Chapter 6, specifically Section 6.5.4 and Chapter 7, specifically Taken into account. -

Reciprocal Museum List
RECIPROCAL MUSEUM LIST DIA members at the Affiliate level and above receive reciprocal member benefits at more than 1,000 museums and cultural institutions in the U.S. and throughout North America, including free admission and member discounts. This list includes organizations affiliated with NARM (North American Reciprocal Museum) and ROAM (Reciprocal Organization of American Museums). Please note, some museums may restrict benefits. Please contact the institution for more information prior to your visit to avoid any confusion. UPDATED: 10/28/2020 DIA Reciprocal Museums updated 10/28/2020 State City Museum AK Anchorage Anchorage Museum at Rasmuson Center AK Haines Sheldon Museum and Cultural Center AK Homer Pratt Museum AK Kodiak Kodiak Historical Society & Baranov Museum AK Palmer Palmer Museum of History and Art AK Valdez Valdez Museum & Historical Archive AL Auburn Jule Collins Smith Museum of Fine Art AL Birmingham Abroms-Engel Institute for the Visual Arts (AEIVA), UAB AL Birmingham Birmingham Civil Rights Institute AL Birmingham Birmingham Museum of Art AL Birmingham Vulcan Park and Museum AL Decatur Carnegie Visual Arts Center AL Huntsville The Huntsville Museum of Art AL Mobile Alabama Contemporary Art Center AL Mobile Mobile Museum of Art AL Montgomery Montgomery Museum of Fine Arts AL Northport Kentuck Museum AL Talladega Jemison Carnegie Heritage Hall Museum and Arts Center AR Bentonville Crystal Bridges Museum of American Art AR El Dorado South Arkansas Arts Center AR Fort Smith Fort Smith Regional Art Museum AR Little Rock -

SJMA Members at the $75 Level and Above Can Enjoy Benefits at the Following Museums: Western Museum Group (WMG)
Reciprocal Membership Privileges: Museum members at the Dual/Family ($75) level and above receive reciprocal privileges at museums affiliated with the Western Museum Group (WMG). Those at the Advocate ($150) level and above also receive reciprocal privileges at museums in both the Museum Alliance Reciprocal Program (MARP), Reciprocal Organization of Associated Museums (ROAM) and also the North American Reciprocal Membership (NARM) programs. Please check with institution for their reciprocity policy. SJMA Members at the $75 level and above can enjoy benefits at the following museums: Western Museum Group (WMG) California Museum of Craft and Folk Art, SF Santa Barbara Museum of Art Other Western States Carnegie Art Museum, Oxnard Museum of Photographic Arts, San Diego Seymour Marine Discovery Center Bellevue Art Museum, WA Fresno Art Museum National Steinbeck Center The Museum of Art & History, Santa Cruz Missoula Art Museum, Montana Fresno Metropolitan Museum Orange County Museum of Art UCR California Museum of Photography Phoenix Art Museum, AZ Long Beach Museum of Art Pacific Asia Museum, Pasadena University Art Museum, Santa Barbara Tucson Museum of Art and Historic Block, AZ Museum of Contemporary Art, San Diego & LaJolla San Jose Museum of Quilts and Textiles The Contemporary Museum, Honolulu SJMA Members at the $150 level and above can also enjoy benefits at the following museums: Museum Alliance Reciprocal Program (MARP) North American Reciprocal Membership (NARM) Reciprocal Organization of Associated Museums (ROAM) Alaska San Diego -

Climate Change: Evidence & Causes 2020
Climate Change Evidence & Causes Update 2020 An overview from the Royal Society and the US National Academy of Sciences n summary Foreword CLIMATE CHANGE IS ONE OF THE DEFINING ISSUES OF OUR TIME. It is now more certain than ever, based on many lines of evidence, that humans are changing Earth’s climate. The atmosphere and oceans have warmed, which has been accompanied by sea level rise, a strong decline in Arctic sea ice, and other climate-related changes. The impacts of climate change on people and nature are increasingly apparent. Unprecedented flooding, heat waves, and wildfires have cost billions in damages. Habitats are undergoing rapid shifts in response to changing temperatures and precipitation patterns. The Royal Society and the US National Academy of Sciences, with their similar missions to promote the use of science to benefit society and to inform critical policy debates, produced the original Climate Change: Evidence and Causes in 2014. It was written and reviewed by a UK-US team of leading climate scientists. This new edition, prepared by the same author team, has been updated with the most recent climate data and scientific analyses, all of which reinforce our understanding of human-caused climate change. The evidence is clear. However, due to the nature of science, not every detail is ever totally settled or certain. Nor has every pertinent question yet been answered. Scientific evidence continues to be gathered around the world. Some things have become clearer and new insights have emerged. For example, the period of slower warming during the 2000s and early 2010s has ended with a dramatic jump to warmer temperatures between 2014 and 2015. -

North Americanreciprocal Museum(NARM)
Table of Contents BERMUDA .................. 1 CANADA ..................... 1 EL SALVADOR ........... 1 UNITED STATES ........ 1 ALABAMA ................... 1 ALASKA ...................... 1 ARIZONA .................... 1 ARKANSAS ................ 1 CALIFORNIA .............. 1 COLORADO ............... 3 CONNECTICUT .......... 3 DELAWARE ................ 4 D.C. ............................ 4 FLORIDA .................... 4 GEORGIA ................... 5 HAWAII ....................... 5 IDAHO ........................ 5 North American Reciprocal ILLINOIS ..................... 6 INDIANA ..................... 6 Museum (NARM) Association IOWA .......................... 6 KANSAS ..................... 6 KENTUCKY ................ 6 LOUISIANA ................. 6 MAINE ........................ 7 Winter 2015-2016 MARYLAND ................ 7 MASSACHUSETTS .... 7 MICHIGAN .................. 8 MINNESOTA............... 8 MISSISSIPPI............... 8 MISSOURI .................. 8 MONTANA .................. 8 NEBRASKA ................ 8 NEVADA ..................... 9 NEW HAMPSHIRE ..... 9 NEW JERSEY ............. 9 NEW MEXICO ............ 9 NEW YORK ................ 9 NORTH CAROLINA .. 10 NORTH DAKOTA ..... 11 OHIO ........................ 11 OKLAHOMA ............. 11 OREGON .................. 11 PENNSYLVANIA ...... 11 RHODE ISLAND ....... 12 SOUTH CAROLINA .. 12 SOUTH DAKOTA ...... 12 TENNESSEE ............ 12 TEXAS ...................... 12 UTAH ........................ 13 VERMONT ................ 13 VIRGINIA .................. 13 WASHINGTON ........ -

Appendix A: Educational Resources in Astronomy
Appendix A: Educational Resources in Astronomy A.I Planetariums, Museums, and Exhibits A.I.I Planetariums and Museums in the United Kingdom England - AAC Planetarium, Amateur Astronomy Centre, Bacup Road, Clough Bank, Tod morden, Lancs. OLl4 7HW. Tel: 0706816964. - British Museum, Great Russell Street, London WC1B 3DG; Tel: 071-323 8395 ext. 395. Astronomical clocks. - British Museum (Natural History), Cromwell Road, South Kensington, London SW7 5BD; Tel: 071-938 9123. Extensive meteorite collection. - Caird Planetarium, Old Royal Observatory, Greenwich, London SE 10. - William Day Planetarium, Plymouth Polytechnic, School of Maritime Studies, Ply- mouth PL4 8AA. Tel: 0752 264666. - Electrosonic Ltd., 815 Woolwich Road, London SE7 8LT. - Greenwich Planetarium, South Building, Greenwich Park, Greenwich, London SE 10. Tel: 081-858 1167. - William Herschel House and Museum, 19 New King Street, Bath, BA1 2Bl. Con tact: Dr. A.V. Sims, 30 Meadow Park, Bathford, Bath; Tel: 0225 859529. Open Mar-Oct daily 2-5 pm, Nov-Feb Sundays only, 2-5 pm. - lodrell Bank Planetarium and Visitor Center, Lower Withington, Nr. Macclesfield, Cheshire SK11 9DL; Tel: 0477 71339. - Kings Observatory, Kew, Old Deer Park, Richmond, Surrey TW9 2AZ. - University of Leicester, The Planetarium, Department of Astronomy, University Road, Leicester LEI 7RH; Tel: 0533 522522. - Liverpool Museum Planetarium, William Brown Street, Liverpool, Merseyside L3 8EN. Tel: 051-2070001 ext. 225. - London Planetarium, Marylebone Road, London NW1 5LR; Tel: 071-486 1121 (9:30--5:30), 071-486 1121 (recording). - City of London Polytechnic, The Planetarium, 100 Minories, Tower Hill, London EC3N BY. 071-283 1030. - London Schools Planetarium, John Archer School Building, Wandsworth Rd., Sutherland Grove, London SW18; Tel: 081-788 4253.