Science, Volume 1, Number 1, November 2012 RESEARCH
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thiruvallur District
DISTRICT DISASTER MANAGEMENT PLAN FOR 2017 TIRUVALLUR DISTRICT tmt.E.sundaravalli, I.A.S., DISTRICT COLLECTOR TIRUVALLUR DISTRICT TAMIL NADU 2 COLLECTORATE, TIRUVALLUR 3 tiruvallur district 4 DISTRICT DISASTER MANAGEMENT PLAN TIRUVALLUR DISTRICT - 2017 INDEX Sl. DETAILS No PAGE NO. 1 List of abbreviations present in the plan 5-6 2 Introduction 7-13 3 District Profile 14-21 4 Disaster Management Goals (2017-2030) 22-28 Hazard, Risk and Vulnerability analysis with sample maps & link to 5 29-68 all vulnerable maps 6 Institutional Machanism 69-74 7 Preparedness 75-78 Prevention & Mitigation Plan (2015-2030) 8 (What Major & Minor Disaster will be addressed through mitigation 79-108 measures) Response Plan - Including Incident Response System (Covering 9 109-112 Rescue, Evacuation and Relief) 10 Recovery and Reconstruction Plan 113-124 11 Mainstreaming of Disaster Management in Developmental Plans 125-147 12 Community & other Stakeholder participation 148-156 Linkages / Co-oridnation with other agencies for Disaster 13 157-165 Management 14 Budget and Other Financial allocation - Outlays of major schemes 166-169 15 Monitoring and Evaluation 170-198 Risk Communications Strategies (Telecommunication /VHF/ Media 16 199 / CDRRP etc.,) Important contact Numbers and provision for link to detailed 17 200-267 information 18 Dos and Don’ts during all possible Hazards including Heat Wave 268-278 19 Important G.Os 279-320 20 Linkages with IDRN 321 21 Specific issues on various Vulnerable Groups have been addressed 322-324 22 Mock Drill Schedules 325-336 -

Table T2.1: Distribution of Participants Across Study Villages and Municipal Zones Within Chosen Study Districts
Table T2.1: Distribution of participants across study villages and municipal zones within chosen study districts Site of No of No of Recruitment (PHC) Participants Participants Village (for pregnant mothers) (M-C Cohort) (Adult Cohort) Agaram Vidayur 2 Agarammel Thirumazhisai 1 Alinjivakkam Perambakkam 1 Anaikattucherry Sorancheri 7 6 Aranvoyal Perumalpattu 5 8 Chembarambakkam Poonamallee 9 9 Chettipedu Thirumazhisai 1 Chinnamangadu Poonamallee 3 Chitharaiambakkam Perambakkam 6 Egathur Kadambathur 1 Goodapakkam, Kuchikadu, Nayapakkam Nemam 18 7 Gundumedu Thirumazhisai 13 5 Ikkadu 17 Ikkadukandiagi 16 Irulanjeri Perambakkam 7 8 Iyyappanthangal SRU 1 Kannur Ulundhai 4 4 Karaiyanchavadi Poonamallee 24 Karanai Vidayur 1 Kattupakkam Poonamallee 36 Kavalchery Soranjeri 1 kavankulathur Perambakkam 2 Keezhmanambedu Thirumazhisai 11 11 Kilacheri Perambakkam 5 Kolappanjeri Poonamallee 3 Kollumedu(Avadi) Poonamallee 2 Kondanjeri Perambakkam 4 2 Kottaiyur 2 Koovum Perambakkam 1 Kumananchavadi Poonamallee 16 Kundrathur Kundrathur 1 11 Kuthampakkam, Irulapalayam, Pappanchathiram,Utkottai, Samathuvapuram Nemam 21 Madavilagam Thirumazhisai 3 Mangadu Poonamallee 4 19 Mappedu Ulundhai 2 Meiyur Nemam 2 1 Melmanambedu 3 Meppur Thirumazhisai 1 Meppurthangal Thirumazhisai 4 Mevalurkuppam(Thandalam) Perambakkam 1 Nagarthangal 3 Narasingapuram Thirumazhisai 3 1 Nazrathpet Thirumazhisai 12 5 Nedunchery Thirumazhisai 2 2 Nemam, Andarasanpettai Nemam 8 Nochimedu Nemam 1 Orathur Thiruvalangadu 1 15 Paarivakkam Poonamallee 15 5 Padur Nemam 5 3 Pappanchatram 3 Panapakkam -
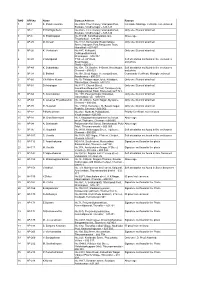
SNO APP.No Name Contact Address Reason 1 AP-1 K
SNO APP.No Name Contact Address Reason 1 AP-1 K. Pandeeswaran No.2/545, Then Colony, Vilampatti Post, Intercaste Marriage certificate not enclosed Sivakasi, Virudhunagar – 626 124 2 AP-2 P. Karthigai Selvi No.2/545, Then Colony, Vilampatti Post, Only one ID proof attached. Sivakasi, Virudhunagar – 626 124 3 AP-8 N. Esakkiappan No.37/45E, Nandhagopalapuram, Above age Thoothukudi – 628 002. 4 AP-25 M. Dinesh No.4/133, Kothamalai Road,Vadaku Only one ID proof attached. Street,Vadugam Post,Rasipuram Taluk, Namakkal – 637 407. 5 AP-26 K. Venkatesh No.4/47, Kettupatti, Only one ID proof attached. Dokkupodhanahalli, Dharmapuri – 636 807. 6 AP-28 P. Manipandi 1stStreet, 24thWard, Self attestation not found in the enclosures Sivaji Nagar, and photo Theni – 625 531. 7 AP-49 K. Sobanbabu No.10/4, T.K.Garden, 3rdStreet, Korukkupet, Self attestation not found in the enclosures Chennai – 600 021. and photo 8 AP-58 S. Barkavi No.168, Sivaji Nagar, Veerampattinam, Community Certificate Wrongly enclosed Pondicherry – 605 007. 9 AP-60 V.A.Kishor Kumar No.19, Thilagar nagar, Ist st, Kaladipet, Only one ID proof attached. Thiruvottiyur, Chennai -600 019 10 AP-61 D.Anbalagan No.8/171, Church Street, Only one ID proof attached. Komathimuthupuram Post, Panaiyoor(via) Changarankovil Taluk, Tirunelveli, 627 761. 11 AP-64 S. Arun kannan No. 15D, Poonga Nagar, Kaladipet, Only one ID proof attached. Thiruvottiyur, Ch – 600 019 12 AP-69 K. Lavanya Priyadharshini No, 35, A Block, Nochi Nagar, Mylapore, Only one ID proof attached. Chennai – 600 004 13 AP-70 G. -
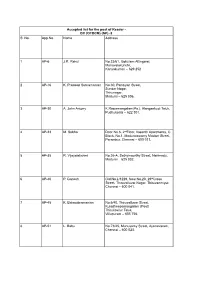
S. No. App.No. Name Address 1 AP-6 J.R. Rahul 2 AP-16 K. Pradeep
Accepted list for the post of Reader - BC (OTBCM) (NP) -2 S. No. App.No. Name Address 1 AP-6 J.R. Rahul No.23/61, Gokulam Attingarai, Manavalakurichi, Kanyakumari – 629 252 2 AP-16 K. Pradeep Subramanian No.30, Pandiyan Street, Sundar Nagar, Thirunagar, Madurai – 625 006. 3 AP-30 A. John Antony K.Rasiamangalam(Po.), Alangankudi Taluk, Pudhukottai – 622 301. 4 AP-33 M. Subha Door No.6, 2ndFloor, Vasanth Apartments, C Block, No.1, Maduraiswamy Madam Street, Perambur, Chennai – 600 011. 5 AP-35 R. Vijayalakshmi No.26-A, Sathymoorthy Street, Narimedu, Madurai – 625 002. 6 AP-40 P. Ganesh Old No.L/1229, New No.20, 29thCross Street, Thiruvalluvar Nagar, Thiruvanmiyur, Chennai – 600 041. 7 AP-45 K. Balasubramanian No.6/40, Thiruvalluvar Street, Kuladheepamangalam (Post) Thirukovilur Taluk, Villupuram – 605 756. 8 AP-51 L. Babu No.73/45, Munusamy Street, Ayanavaram, Chennai – 600 023. 9 AP-56 S. Barkavi No.168, Sivaji Nagar, Veerampattinam, Pondicherry – 605 007. 10 AP-62 R.D. Mathanram No.57, Jeeyar Narayanapalayam St, Kanchipuram – 631 501 11 AP-77 M.Parameswari No.8/4, Alagiri Nagar, 1ststreet, Vadapalani, chennai -26. 12 AP-83 G. Selva Kumari No. 12, G Block, Singara thottam, Police Quarters, Old Washermen pet, Chennai 600 021 13 AP-89 P. Mythili No.137/64, Sanjeeviroyan Koil Street, Old Washermenpet, Chennai – 600 021. 14 AP-124 K. Balaji No.11, Muthumariamman Koil Street, Bharath Nagar, Selaiyur, Chennai – 600 073. 15 AP-134 S. Anitha No.5/55-A, Main Road, Siruvangunam, Iraniyasithi Post, Seiyur Taluk, Kancheepuram – 603 312. -
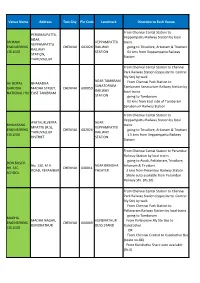
Venue Name Address Test City Pin Code Landmark Direction to Each Venue
Venue Name Address Test City Pin Code Landmark Direction to Each Venue From Chennai Cental Station to PERUMALPATTU, Veppampattu Railway Station by local NEAR SRI RAM VEPPAMPATTU trains VEPPAMPATTU ENGINEERING CHENNAI 602024 RAILWAY going to Trivallore, Arkonam & Tiruttani. RAILWAY COLLEGE STATION 01 kms from Veppampattu Railway STATION, Station THIRUVALLUR From Chennai Cental Station to Chennai Park Railway Station (opposite to Central Rly Stn) by walk NEAR TAMBRAM JAI GOPAL BHARADHA From Chennai Park Station to SANATORIUM GARODIA MADHA STREET, CHENNAI 600059 Tambaram Sanatorium Railway Station by RAILWAY NATIONAL HSS EAST TAMBRAM local trains STATION going to Tambaram 01 kms from East side of Tambaram Sanatorium Railway Station From Chennai Cental Station to Veppampattu Railway Station by local AYATHUR,VEPPA NEAR BHAJARANG trains MPATTU (R.S), VEPPAMPATTU ENGINEERING CHENNAI 602024 going to Trivallore, Arkonam & Tiruttani. THIRUVALLUR RAILWAY COLLEGE 1.5 kms from Veppampattu Railway DISTRICT STATION Station From Chennai Cental Station to Perambur Railway Station by local trains going to Avadi, Pattabiram,Trivallore, DON BOSCO No. 130, M.H. NEAR BRINDHA Arkonam & Tiruttani. HR. SEC. CHENNAI 600011 ROAD, PERAMBUR THEATER 2 kms from Perambur Railway Station SCHOOL Share auto available from Perambur Railway Stn. (Rs.10) From Chennai Cental Station to Chennai Park Railway Station (opposite to Central Rly Stn) by walk From Chennai Park Station to Pallavaram Railway Station by local trains going to Tambaram MADHA MADHA NAGAR, KUNDRATHUR From Pallavaram Rly Stn Bus to ENGINEERING CHENNAI 600069 KUNDRATHUR BUSS STAND Kundrathur. COLLEGE OR From Chennai Central to Kundrathur Bus (route no.88) From Kundrathu Share auto available (Rs.5) Dr. -

Nodal Contacts
BHARAT SANCHAR NIGAM LIMITED O/o PGM S, 40-E, CIPET ROAD TVK INDUSTRIAL ESTATE, GUINDY-32 LR.NO: PGM/S/2019-20/ENLISTING FTTH VENDORS/ DT 02.05.2019 Sub: Enlisting of FTTH Partners with BSNL UnderRevenue Share Business Model-reg. BSNL, Chennai Telephones, is in the Process of Enlisting New Vendor Agencies for providing Voice & Broadband Services thru Optical Fibre Cable Connectivity to Individual Homes (FTTH) on Revenue Share Basis (50:50) Interested Agencies with Valid License / Minimum One Year Experience are requested to submit their willingness for Entering into Business Agreement with BSNL in this regard. Eligibility Criteria: 1. Registered Builder 2. Registered Residential Welfare Association 3. Local Cable Operators (LCOs) / Multi Service Operators (MSOs) with valid license 4. Infrastructure Providers-1 (IP-1) / Virtual Network Operators (VNOs) with valid license 5. Existing Cable Operators, Firms working for OFC laying, Broadband Provisioning & Maintenance and other firms working in Telecom field etc. (A company incorporated under the company Act 1956, or Proprietorship/ Partnership firms) Coverage Area: Exchanges / Postal Pincodes, Covered under South and West Areas inChennai Telephones District, as Annexed. Nodal contacts I. South Business Area No. Name Designation Mobile No. Mail ID 1 A.Kalaiselvi AGM 9444979100 2 R.Petchimuthu SDE 9444995599 [email protected] 3 S.Mathiprakasam SDE 9444971900 4 D.Aravind SDE 9444040368 II. West Business Area No. Name Designation Mobile No. Mail ID 1 ShanthiRamani AGM 9445395353 2 Chandra -

I Typewriting Institutions Renewal Approval - 2021 EXTENSION of SL
DIRECTORATE OF TECHNICAL EDUCATION, CHENNAI-600 025. Phase - I Typewriting Institutions Renewal Approval - 2021 EXTENSION OF SL. APPROVAL COURSE APPROVED FOR INSTITUTE NAME INSTITUTE ADDRESS PROPRIETOR NAME APPROVAL GIVEN NO. NO. CONDUCTING CLASSES UPTO SRI RAM COMPLEX, DOOR NO 7 BHARATHI 1 250712 JAYA TYPEWRITING INSTITUTE STREET, SRI RAM NAGAR KOTTAIYUR NARAYANAN V 1, 2, 11, 12, 21, 22 2021 SIVAGANGAI- 630106 6/215 P, NETHAJI ROAD, PARAMAKUDI, 2 250715 SRI KRISHNA TYPEWRITING INSTITUTE KRISHNAVENI C 1, 2, 11, 12, 21, 22 2021 RAMANATHAPURAM- 623707 17/136, MIDDLE STREET, RAMESWARAM 3 250717 S M S TYPEWRITING INSTITUTE SHANTHI K 1, 2, 11, 12, 21, 22 2021 RAMANATHAPURAM- 623526 NO. 8/356, KALLAZHAGAR STREET, 4 233037 SRI RAM TECHNICAL INSTITUTE PARAMAKUDI, RAMANATHAPURAM - DHANASEKARAN P 1, 2, 11, 12, 21, 22 2021 623707 NO:27/11, SIVAN KOVIL WEST STREET, 5 250109 VASAN'S TYPEWRITING INSTITUTE UMAYAMBAL RAMANATHAN 1, 2, 11, 12, 21, 22 2021 SWARNAMANI NIVAS,SIVAGANGAI- 630561 NO:33, AGRAHARAM STREET, KOTTAI 6 250123 SRI ARAVIND SCHOOL OF COMMERCE GOVINDHARAJAN K 1, 2, 11, 12, 21, 22 2021 THIRUBUVANAM,SIVAGANGAI- 630611 KALLAL ROAD, KALAYAR KOVIL TK, 7 250154 SELES TYPEWRITING INSTITUTE VINCENT S 1, 2, 11, 12, 21, 22 2021 SIVAGANGAI- 630551 OLD, NO10A, NEW NO. 3, MADHA KOVIL 8 250201 BHILAL TYPEWRITING SCHOOL MITHARNISSAL BEEVI K S A 1, 2, 11, 12, 21, 22 2021 STREET, ILAYANGUDI,SIVAGANGAI- 630702 NO:13, PALACE ROAD, RAMANATHAPURAM, 9 250205 VASAN’S TYPEWRITING INSTITUTE SUTHA 1, 2, 11, 12, 21, 22 2021 RAMANATHAPURAM- 623501 NO:12 AGRAHARAM -

Tamil Nadu Government Gazette
© [Regd. No. TN/CCN/467/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2013 [Price: Rs. 24.80 Paise. TAMIL NADU GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY No. 5] CHENNAI, WEDNESDAY, FEBRUARY 6, 2013 Thai 24, Nandhana, Thiruvalluvar Aandu–2044 Part VI—Section 4 Advertisements by private individuals and private institutions CONTENTS PRIVATE ADVERTISEMENTS Pages Change of Names .. 239-298 Notices .. 299 NOTICE NO LEGAL RESPONSIBILITY IS ACCEPTED FOR THE PUBLICATION OF ADVERTISEMENTS REGARDING CHANGE OF NAME IN THE TAMIL NADU GOVERNMENT GAZETTE. PERSONS NOTIFYING THE CHANGES WILL REMAIN SOLELY RESPONSIBLE FOR THE LEGAL CONSEQUENCES AND ALSO FOR ANY OTHER MISREPRESENTATION, ETC. (By Order) Director of Stationery and Printing. CHANGE OF NAMES 3594. My son, A. Sengol Arokiya Beneto, son of 3597. I, S. Georgeford Thangadurai, son of Thiru Thiru S. Arockiasamy, born on 22nd March 2002 (native V. Suviseshamuthu, born on 7th September 1951 district: Sivagangai), residing at No. 3/55, Nedodai, (native district: Thoothukkudi), residing at No. 49, State Puliyadithambam Post, Sivagangai-630 411, shall henceforth Bank 1st Colony, Bye Pass Road, Madurai-625 016, shall be known as A BENITTO. henceforth be known as S. GEORGE FORD. S. GEORGEFORD THANGADURAI. A. STELLA RANI. Madurai, 28th January 2013. Sivagangai, 28th January 2013. (Mother.) 3598. I, R. Petchiammal alias Meena, wife of 3595. My son, P. Aravind, born on 7th November 1995 Thiru G. Mani, born on 10th May 1965 (native district: (native district: Madurai), residing at 3/467, Maruthi Nagar, Tirunelveli), residing at Old No. 9A, New No. 13, Murugan Madurai Road, Usilampatti Taluk, Madurai-625 532, shall Talkies, 4th Cross Street, Thiruvalluva Nagar, henceforth be known as P. -

Tamil Nadu Government Gazette
© [Regd. No. TN/CCN/467/2009-11. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2010 [Price: Rs. 23.20 Paise. TAMIL NADU GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY No. 27] CHENNAI, WEDNESDAY, JULY 14, 2010 Aani 30, Thiruvalluvar Aandu–2041 Part VI—Section 4 Advertisements by private individuals and private institutions CONTENTS PRIVATE ADVERTISEMENTS Pages Change of Names .. 1259-1316 Notice .. 1316 NOTICE NO LEGAL RESPONSIBILITY IS ACCEPTED FOR THE PUBLICATION OF ADVERTISEMENTS REGARDING CHANGE OF NAME IN THE TAMIL NADU GOVERNMENT GAZETTE. PERSONS NOTIFYING THE CHANGES WILL REMAIN SOLELY RESPONSIBLE FOR THE LEGAL CONSEQUENCES AND ALSO FOR ANY OTHER MISREPRESENTATION, ETC. (By Order) Director of Stationery and Printing. CHANGE OF NAMES My son, P. Manoj, born on 8th October 1996 (native My daughter, R. Harini, daughter of Thiru A.S. Ranganathan, district: Erode), residing at Old No. 2/26, New No. 1/79, born on 15th December 1993 (native district: Thiruvannamalai), Kongampalayam, Chittode, Erode-638 102, shall hencefroth residing at No. 122, Bharathi Street, V.G.P. Shanthi Nagar, be known as P. METHUNRAJ. Narayanapuram, Chennai-600 100, shall henceforth be known as R. SRIHARINI. K.R.E. PONGI. Chittode, 5th July 2010. (Father.) RAJALAKSHMI RANGAN. Chennai, 5th July 2010. (Mother.) My son, P. Yaswanth, born on 14th October 1999 (native I, M.C. Deepa, wife of Thiru Bhuvanesh Srinivasan, born district: Erode), residing at Old No. 2/26, New No. 1/79, on 21st May 1977 (native district: Kancheepuram), residing Kongampalayam, Chittode, Erode-638 102, shall henceforth at Old No. 85, New No. 34, Gengu Reddy Street, be known as K.E.P. -

Mint Building S.O Chennai TAMIL NADU
pincode officename districtname statename 600001 Flower Bazaar S.O Chennai TAMIL NADU 600001 Chennai G.P.O. Chennai TAMIL NADU 600001 Govt Stanley Hospital S.O Chennai TAMIL NADU 600001 Mannady S.O (Chennai) Chennai TAMIL NADU 600001 Mint Building S.O Chennai TAMIL NADU 600001 Sowcarpet S.O Chennai TAMIL NADU 600002 Anna Road H.O Chennai TAMIL NADU 600002 Chintadripet S.O Chennai TAMIL NADU 600002 Madras Electricity System S.O Chennai TAMIL NADU 600003 Park Town H.O Chennai TAMIL NADU 600003 Edapalayam S.O Chennai TAMIL NADU 600003 Madras Medical College S.O Chennai TAMIL NADU 600003 Ripon Buildings S.O Chennai TAMIL NADU 600004 Mandaveli S.O Chennai TAMIL NADU 600004 Vivekananda College Madras S.O Chennai TAMIL NADU 600004 Mylapore H.O Chennai TAMIL NADU 600005 Tiruvallikkeni S.O Chennai TAMIL NADU 600005 Chepauk S.O Chennai TAMIL NADU 600005 Madras University S.O Chennai TAMIL NADU 600005 Parthasarathy Koil S.O Chennai TAMIL NADU 600006 Greams Road S.O Chennai TAMIL NADU 600006 DPI S.O Chennai TAMIL NADU 600006 Shastri Bhavan S.O Chennai TAMIL NADU 600006 Teynampet West S.O Chennai TAMIL NADU 600007 Vepery S.O Chennai TAMIL NADU 600008 Ethiraj Salai S.O Chennai TAMIL NADU 600008 Egmore S.O Chennai TAMIL NADU 600008 Egmore ND S.O Chennai TAMIL NADU 600009 Fort St George S.O Chennai TAMIL NADU 600010 Kilpauk S.O Chennai TAMIL NADU 600010 Kilpauk Medical College S.O Chennai TAMIL NADU 600011 Perambur S.O Chennai TAMIL NADU 600011 Perambur North S.O Chennai TAMIL NADU 600011 Sembiam S.O Chennai TAMIL NADU 600012 Perambur Barracks S.O Chennai -

General Properties
8 MYLAPORE TIMES August 23 - 29, 2014 ▲ ▲ l l l l General Properties ll ACCOUNTANCY LEARN 2 Wheeler Ladies teach Ladies We REAL ESTATE / BUYING llSATHOME Behind Montfort School, Rosary llR.A.PURAM MRC Nagar Jains 1345 sq.ft, ll will come at your place. Contact: B. Priya Ph: ACCOUNT, Audit, Vat, Service Tax, TDS, ll Church Lane, Two Bedroom Apartment, 2 BHK Sea view pool, gym, play area, rental 99520 34991 / 90031 73394. WANTED Outright Buy lease Small Flat Income Tax, E-filing. Contact: 97907 41271. 30 Lakhs Immediate Settlement Mylapore, 800sq.ft, First Floor, Lift, Car Park, Esssar yield Rs.60000/- pm Rs.15000/- sq.ft, Call: llMARINA Murthy Driving School Since Mandaveli, R.A. Puram, Alwarpet, F. Estate, Realtor. Ph: 90031 54298. 98849 58743. - 1955 Learn Two / Four wheelers ladies Triplicane Old Flat Ok, Good Water. Ph: llSRIPERUMBUR Govt approved plots per llABHIRAMAPURAM 3 BR Deluxe flat teach ladies No:160, Luz Church Road near CATERING 94447 43397. sq.ft Rs.650/- near Green Field Airport & 1650 sq.ft, Gym / Play area / Genset / CCP Alwarpet Signal. Ph: 98410 20502 / 98414 llSAI Bhojan (Add Taste to your food ll NH4 Plot Size 1000sq.ft to 2400sq.ft. Sun Rs.2.50 Crore / East Abhiramapuram New 3 04888. SELL your Vacant plot in and around undertaking orders for chapattis, phulkas, Keelkatalai, Puzuthivakkam, Nanmangalam, Team Housing Pvt Ltd. Tamilvanan. Ph: BR 1500 sq.ft, Genset CCP Rs.2.30 Crore / llLADIES Driving Two / Four wheelers, for subji and curd rice for dinner. Contact: 98410 Irumbuliyur, Urapakkam, Guduvanchery, 98409 49097. Kottur Garden Appasamy flat 3 BR 1280 sq.ft, ladies only. -

Registered Engineer Grade II
Works Department 30.04.2021 CMDA UPDATING OF REGISTERED ENGINEER LIST 1I - 2019-2020,2020-2021,2021-2022 Education Experience SLNO NAME REG No ADDRESS GRADE PHONE NO EMAIL ID Qualification in years Plot No:172/173, A1, Vallalar Street, Panner Nagar, 1 Murthy.V RE200012019 II 9841246839 [email protected] B.E(Civil Engg.) 09years Mogappair, Chennai-600 037 2 Nagarajan P RE200022019 No: 19/19, Periyar Salai, Ayanavaram, Chennai 600 023. II 9445968274 [email protected] B.E(Civil Engg.) 6years No: 100/2, Kodambakkam Road, Mettupalayam, West B.Tech(Civil 3 Jeyaraj.A RE200032019 II 9080128710 [email protected] 5years Mambalam, Chennai 600 033 Engg.) Plot no: 9, RKR Nagar, 2nd Street, Poompuhar nagar, 1st 4 Suresh Kumar.K RE200042019 II 9841088565 [email protected] B.E(Civil Engg.) 5years Main Road, Kolathur, Chennai 600 099. B.E(Civil No: 1, 3rd Cross Sastri Street, Kaveri Nagar, Saidapet, 5 Kalaivani.A RE200052019 II 9443734545 [email protected] &Environmental 8years Chennai 600 015. Engg.) A.P. 301, 40th Street, 8th Sector, K.K.Nagar, 6 Kumaresan.T RE200062019 II 9841164342 [email protected] B.E(Civil Engg.) 5years Chennai 600 078 No: 15, 7th Street, L Block, 12th Main Road, Anna Nagar, B.Tech(Civil 7 Venkatesan.M RE200072019 II 9841919096 [email protected] 8years Chennai 600 040. Engg.) No:Plot No:39/A, 4th West Street, Indira Nagar, 8 Niresh Babu.G RE200082019 II 9941198263 [email protected] B.E(Civil Engg.) 9years Kattupakkam, Chennai-600 056 No:23/16, Sasthiri Nagar, 2nd Street, Villivakkam,