Bone Growth Stimulators - Electric
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Medical Policy Ultrasound Accelerated Fracture Healing Device
Medical Policy Ultrasound Accelerated Fracture Healing Device Table of Contents Policy: Commercial Coding Information Information Pertaining to All Policies Policy: Medicare Description References Authorization Information Policy History Policy Number: 497 BCBSA Reference Number: 1.01.05 Related Policies Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures, #498 Electrical Bone Growth Stimulation of the Appendicular Skeleton, #499 Bone Morphogenetic Protein, #097 Policy Commercial Members: Managed Care (HMO and POS), PPO, and Indemnity Members Low-intensity ultrasound treatment may be MEDICALLY NECESSARY when used as an adjunct to conventional management (i.e., closed reduction and cast immobilization) for the treatment of fresh, closed fractures in skeletally mature individuals. Candidates for ultrasound treatment are those at high risk for delayed fracture healing or nonunion. These risk factors may include either locations of fractures or patient comorbidities and include the following: Patient comorbidities: Diabetes, Steroid therapy, Osteoporosis, History of alcoholism, History of smoking. Fracture locations: Jones fracture, Fracture of navicular bone in the wrist (also called the scaphoid), Fracture of metatarsal, Fractures associated with extensive soft tissue or vascular damage. Low-intensity ultrasound treatment may be MEDICALLY NECESSARY as a treatment of delayed union of bones, including delayed union** of previously surgically-treated fractures, and excluding the skull and vertebra. 1 Low-intensity ultrasound treatment may be MEDICALLY NECESSARY as a treatment of fracture nonunions of bones, including nonunion*** of previously surgically-treated fractures, and excluding the skull and vertebra. Other applications of low-intensity ultrasound treatment are INVESTIGATIONAL, including, but not limited to, treatment of congenital pseudarthroses, open fractures, fresh* surgically-treated closed fractures, stress fractures, arthrodesis or failed arthrodesis. -

CASE REPORT Injuries Following Segway Personal
UC Irvine Western Journal of Emergency Medicine: Integrating Emergency Care with Population Health Title Injuries Following Segway Personal Transporter Accidents: Case Report and Review of the Literature Permalink https://escholarship.org/uc/item/37r4387d Journal Western Journal of Emergency Medicine: Integrating Emergency Care with Population Health, 16(5) ISSN 1936-900X Authors Ashurst, John Wagner, Benjamin Publication Date 2015 DOI 10.5811/westjem.2015.7.26549 License https://creativecommons.org/licenses/by/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California CASE REPORT Injuries Following Segway Personal Transporter Accidents: Case Report and Review of the Literature John Ashurst DO, MSc Conemaugh Memorial Medical Center, Department of Emergency Medicine, Benjamin Wagner, DO Johnstown, Pennsylvania Section Editor: Rick A. McPheeters, DO Submission history: Submitted April 20, 2015; Accepted July 9, 2015 Electronically published October 20, 2015 Full text available through open access at http://escholarship.org/uc/uciem_westjem DOI: 10.5811/westjem.2015.7.26549 The Segway® self-balancing personal transporter has been used as a means of transport for sightseeing tourists, military, police and emergency medical personnel. Only recently have reports been published about serious injuries that have been sustained while operating this device. This case describes a 67-year-old male who sustained an oblique fracture of the shaft of the femur while using the Segway® for transportation around his community. We also present a review of the literature. [West J Emerg Med. 2015;16(5):693-695.] INTRODUCTION no parasthesia was noted. In 2001, Dean Kamen developed a self-balancing, zero Radiograph of the right femur demonstrated an oblique emissions personal transportation vehicle, known as the fracture of the proximal shaft of the femur with severe Segway® Personal Transporter (PT).1 The Segway’s® top displacement and angulation (Figure). -

Clinical Efficacy of Extended Modified Posteromedial Approach Versus
Clinical ecacy of extended modied posteromedial approach versus posterolateral approach for surgical treatment of posterior pilon fracture: a retrospective analysis Qin-Ming Zhang Aliated Hospital of Jining Medical University Hai-Bin Wang Aliated Hospital of Jining Medical University Xiao-Yan Li Aliated Hospital of Jining Medical University Feng-Long Chu Aliated Hospital of Jining Medical University Liang Han Aliated Hospital of Jining Medical University Dong-Mei Li Aliated Hospital of Jining Medical University Bin Wu ( [email protected] ) Alited Hospital of Jining Medical University https://orcid.org/0000-0003-3707-0056 Research article Keywords: posterior pilon fracture, approach, fracture xation, buttress plate Posted Date: March 30th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-19392/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/12 Abstract Background: Posterior pilon fracture is a type of ankle fracture associated with poorer treatment results compared to the conventional ankle fracture. This is partly related to the lack of consensus on the classication, approach selection, and internal xation method for this type of fracture. This study aimed to investigate the clinical ecacy of posterolateral approach versus extended modied posteromedial approach for surgical treatment of posterior pilon fracture. Methods: Data of 67 patients with posterior pilon fracture who received xation with a buttress plate between January 2015 and December 2018 were retrospectively reviewed. Patients received steel plate xation through either the posterolateral approach (n = 35, group A) or the extended modied posteromedial approach (n = 32, group B). Operation time, intraoperative blood loss, excellent and good rate of reduction, fracture healing time, American Orthopaedic Foot & Ankle Society (AOFAS) Ankle- Hindfoot Scale score, and Visual Analogue Scale score were compared between groups A and B. -

Bimalleolar Ankle Fracture Fixation of a 13-Year-Old Patient with Two Activascrew™ LAG Bioabsorbable Screws
Bimalleolar ankle fracture fixation of a 13-year-old patient with two ActivaScrew™ LAG bioabsorbable screws. Pierre Lascombes Professor, M.D., Ph.D. For medical professional use, not public. © 2020 Bioretec Ltd. and Prof. Lascombes. All rights reserved. Table of Contents Summary Table ........................................................................................................... 3 1 Case Description .................................................................................................. 4 2 Implants and operative technique ......................................................................... 5 3 Outcome ............................................................................................................... 7 4 Contact Information Concerning the Case ............................................................ 8 For medical professional use, not public Page 2 / 8 © 2020 Bioretec Ltd., Prof. Lascombes. All rights reserved. Summary Table Demographics Patient number: P01 Patient Initials: NX Smoking: No Sex: Female Use of alcohol: No Age: 13 Years Systemic disease: No Height: 158 cm Cont. Medication: No Weight: 40 kg Case description Diagnosis: Dislocation right ankle - bimalleolar fracture Cause of injury: Gymnastic injury Operation Operator: Lascombes Operation year: 2016 Note: Immediate reduction of the Operation description: Open reduction of medial malleolus and fixation with TWO resorbable screws dislocation at emergenry room - ketamine. Surgical treatment 5 hours Operation time:h57 min Immobilisation -

OUTPATIENT REHABILITATION for a PATIENT FOLLOWING a SURGICALLY STABILIZED TRIMALLEOLAR FRACTURE a Doctoral Project a Comprehensi
OUTPATIENT REHABILITATION FOR A PATIENT FOLLOWING A SURGICALLY STABILIZED TRIMALLEOLAR FRACTURE A Doctoral Project A Comprehensive Case Analysis Presented to the faculty of the Department of Physical Therapy California State University, Sacramento Submitted in partial satisfaction of the requirements for the degree of DOCTOR OF PHYSICAL THERAPY by Julie Hammond FALL 2017 © 2017 Julie Hammond ALL RIGHTS RESERVED ii OUTPATIENT REHABILITATION FOR A PATIENT FOLLOWING A SURGICALLY STABILIZED TRIMALLEOLAR FRACTURE A Doctoral Project by Julie Hammond Approved by: _____________________________________, Committee Chair Edward Barakatt, PT, PhD _____________________________________, First Reader William Garcia, PT, DPT, OCS, FAAOMPT _____________________________________, Second Reader Rafael Escamilla, PhD, PT, CSCS ____________________________ Date iii Student: Julie Hammond I certify that this student has met the requirements for format contained in the University format manual, and that this project is suitable for shelving in the Library and credit is to be awarded for the project. __________________________________, Department Chair ____________ Michael McKeough, PT, EdD Date Department of Physical Therapy iv Abstract of OUTPATIENT REHABILITATION FOR A PATIENT FOLLOWING A SURGICALLY STABILIZED TRIMALLEOLAR FRACTURE by Julie Hammond A 46 year old woman with a right surgically stabilized trimalleolar fracture was seen for physical therapy treatment for 13 sessions from 06/17/2016 to 09/16/2106 at an outpatient pro bono physical therapy clinic. Treatment was provided by a student physical therapist under the supervision of a licensed physical therapist. The patient was evaluated at the initial encounter with manual muscle test, goniometric measurements, single leg balance test, Timed Up and Go test, and a self- report questionnaire (Foot and Ankle Ability Measure). -

Bimalleolar Fracture of Ankle Joint Managed by Tension Band Wiring Technique: a Prospective Study Dr
Scholars Journal of Applied Medical Sciences (SJAMS) ISSN 2320-6691 (Online) Sch. J. App. Med. Sci., 2014; 2(1D):428-432 ISSN 2347-954X (Print) ©Scholars Academic and Scientific Publisher (An International Publisher for Academic and Scientific Resources) www.saspublisher.com Research Article Bimalleolar Fracture of Ankle Joint Managed By Tension Band Wiring Technique: A Prospective Study Dr. Maruthi CV*1, Dr.Venugopal N2, Dr. Nanjundappa HC2, Dr. Siddalinga swamy MK2 1Assistant Professor, Department of Orthopaedics, MVJ MC and RH, Hoskote, Bangalore, India 2Professor, Dept of Orthopaedics, MVJ MC and RH, Hoskote, Bangalore, India 3MVJ Medical College & Research Hospital, Hoskote, Bangalore -562 114, India *Corresponding author Dr. Maruthi CV Email: Abstract: Ankle fractures are the most commonly encountered by most of the orthopaedic surgeons. According to the lauge Hansen’s classification five different types can be seen. The surgical treatment of adduction, abduction and supination external rotation type of injuries leading to bimalleolar fractures can be fixed with either tension band technique or cancellous screws. Here we are done a study to evaluate the benefits of tension band wiring technique in the management of bimalleolar fractures of the ankle. In our study, 40 cases of bimalleolar fracture of ankle joint of above mentioned types were admitted in Department of Orthopaedics, between February 2009 and November 2013 was included. We included patients above 20 and below 58 years. We excluded patients with pronation external rotation, vertical compression and trimalleolar fractures, pathological fractures, compound fractures and who are medically unfit and at extremely high anaesthesia risk. All the patients, operated by open reduction and internal fixation using tension band wiring technique. -

Risk Factors for Fracture and Fracture Severity of the Distal Radius and Ankle. What About Osteoporosis, Celiac Disease and Obesity?
Risk factors for fracture and fracture severity of the distal radius and ankle. What about osteoporosis, celiac disease and obesity? Anja Myhre Hjelle Thesis for the degree of Philosophiae Doctor (PhD) University of Bergen, Norway 2021 Risk factors for fracture and fracture severity of the distal radius and ankle. What about osteoporosis, celiac disease and obesity? Anja Myhre Hjelle ThesisAvhandling for the for degree graden of philosophiaePhilosophiae doctorDoctor (ph.d (PhD). ) atved the Universitetet University of i BergenBergen Date of defense:2017 17.06.2021 Dato for disputas: 1111 © Copyright Anja Myhre Hjelle The material in this publication is covered by the provisions of the Copyright Act. Year: 2021 Title: Risk factors for fracture and fracture severity of the distal radius and ankle. Name: Anja Myhre Hjelle Print: Skipnes Kommunikasjon / University of Bergen 1 CONTRIBUTORS This work is the result of a collaboration between and funding from The Department of Global Public Health and Primary Care at the University of Bergen, The Department of Rheumatology at the District Hospital of Førde, and The Centre for Health Research in Sogn og Fjordane (a collaborative effort between Førde Health Trust and the health studies department of the Western Norway University College of Applied Sciences). We have also collaborated with The Department of Orthopedic surgery and the Department of Radiology at the District Hospital of Førde, The Departement of Rheumatology at Haukeland University Hospital, Bergen, and The KB Jebsen Coeliac Disease Research Centre, University of Oslo. We are grateful for additional funding received from The Norwegian association for Celiac Disease (Norsk Cøliakiforening) in 2012 and 2015. -

The Michigan Trauma Quality Improvement Program
What Drives your Coding? Diagnoses Kathy Cookman 11:00 What Drives Your Coding? DIAGNOSES Kathy J. Cookman, BS, CSTR, CAISS, EMT-P, FMN CEO – KJ Trauma Consulting, LLC International Technical Coordinator/AIS Course director - AAAM Objectives Identify injuries and correct ICD-10-CM and AIS coding Incorporate education of diagnosis coding Incorporate Anatomy and Physiology Rules for coding, specific to diagnoses identified within scenarios Abstracting: Best Practice Consistency in process Read the details Work concurrently Ask questions/seek clarification Work with CDI team (clinical documentation improvement team) Determine core dataset Assigning ICD-10-CM Use a CURRENT ICD-10-CM coding book Start with the INDEX Find the beginning components of the code Turn to the TABULAR section Complete the code Enter the appropriate code into the trauma registry ICD-10-CM Placeholder “X” Assigning AIS Use the most current AIS Dictionary supported by your trauma registry Find the most appropriate AIS code Enter the code into the trauma registry JJ Krash Transfer – 17 year old boy. Unrestrained passenger seated in the 3rd row. From scene to Level 3 Trauma Center. Transferred to burn center with 8% TBSA burns via ALS ambulance. Diagnoses: 8% TBSA – 3rd degree RLW – circumferential 1st degree RUE – right forearm Hypothermia – 34.9 JJ Krash Develop a method for abstracting data and be consistent in searching cases the same way each time ED Trauma Flow Sheet Drawings “Area of Injury” can be helpful for external skin injury identification, however, be aware that it may be difficult to determine exactly what is noted, where and how complex – always look for more definitive information TBSA Percentage? Extremity Comments = “12% TBSA” Nursing Note Narrative = “12% TBSA” History & Physical = “8% TBSA” What do you do with a discrepancy? JJ Krash Unrestrained passenger seated in the 3rd row of van which lost control, went down a ditch, rolled over and vehicle caught fire. -

Moda Health Plan, Inc. Medical Necessity Criteria
Moda Health Plan, Inc. Subject: Bone Growth Stimulators- Medical Necessity Criteria Ultrasound Page 1 of 8 Origination Date: 01/07 Revision Date(s): 1/08, 1/09, 2/11, 1/12, 09/12, 07/13, 06/14, 09/14, 05/15, 07/15 Developed By: Medical Criteria Committee 01/07 Approved: Mary Engrav, MD Date: 05/27/2015 Description: Bone growth stimulation is a technique of promoting bone growth in difficult to heal fractures. Two types of bone growth stimulators currently exist: electrical and ultrasonic. Ultrasonic fracture healing is a noninvasive treatment that utilizes low intensity, pulsed ultrasound delivered through a skin surface transducer placed directly over the fracture site. It is used to accelerate healing of fresh fractures and fracture nonunions of sites that are difficult to heal because of poor vascular supply. It is frequently used in conjunction with orthopedic treatments such as a cast or splint. The device is portable and can be used by the patient at home in daily 20 minute sessions. The Sonic Accelerated Fracture Healing System (SAFHS) is a type of ultrasound bone growth stimulator. Criteria: I. Ultrasonic Bone Growth Stimulators (CWQI HCS-0008A) A. Ultrasonic bone growth stimulators will be covered to plan limitations for skeletally mature individuals when 1 or more of the following criteria are met: 1. Fresh Acute Fractures a. Most fresh fractures heal with the use of standard fracture care such as closed reduction and cast immobilization. Ultrasonic bone growth stimulators may be indicated for the treatment of fresh fractures when 1 (one) or more of the following risk factors for delayed fracture healing or nonunion are present: i. -

Radiographic Evaluation, Fixation Principles, and Post-Operative Management of Ankle Fractures
Central JSM Foot and Ankle Bringing Excellence in Open Access Review Article *Corresponding author Lisa Zhang, Department of Podiatry, University Hospital, G142. 150 Bergen St. Newark, NJ 07103, USA, Tel: 908- Radiographic Evaluation, 577-2543; Email: Submitted: 15 March 2018 Fixation Principles, and Post- Accepted: 23 June 2017 Published: 25 June 2017 ISSN: 2475-9112 Operative Management of Copyright © 2017 Zhang et al. Ankle Fractures OPEN ACCESS 1 2 Lisa Zhang * and Ritchard Rosen Keywords 1Department of Podiatry, University Hospital, Newark, USA • Fracture blisters 2Department of Podiatric Surgery, Holy Name Medical Center, USA • Neutralization plate • Posterior-antiglide plate • Tension banding Abstract • Direct approach Radiographically, ankle fractures can be evaluated on the anterior-posterior (AP) view • Early weight bearing with the talocrural angle, and on the mortise view, by Shenton’sline, the dime sign, and talar tilt. Displaced fractures > 2 mm, lateral malleolar fractures that are shortened, rotated, or angulated, and bimalleolar and trimalleolar fractures require fixation. Principles of fixation include a proper soft tissue envelope without fracture blisters present at incision sites. Due to the formation of soft callus, fractures should be fixated within the first two weeks of injury. The lateral malleolus is the dominant fracture, and must be pulled out to length, which will pull other ligamentous attachments to proper alignment. Lateral malleolar fixation may be accomplished by a neutralization plate or posterior anti-glide plate; a locking plate may also be used in osteoporotic bone. Medial malleolar fixation can be accomplished by two screw cancellous fixation or tension banding, the latter especially useful in smaller, comminuted fractures. -

Lower Extremity Fracture Eponyms (Part 2) Philip Kin-Wai Wong1, Tarek N Hanna2*, Waqas Shuaib3, Stephen M Sanders4 and Faisal Khosa2
Wong et al. International Journal of Emergency Medicine (2015) 8:25 DOI 10.1186/s12245-015-0076-1 REVIEW Open Access What’s in a name? Lower extremity fracture eponyms (Part 2) Philip Kin-Wai Wong1, Tarek N Hanna2*, Waqas Shuaib3, Stephen M Sanders4 and Faisal Khosa2 Abstract Eponymous extremity fractures are commonly encountered in the emergency setting. Correct eponym usage allows rapid, succinct communication of complex injuries. We review both common and less frequently encountered extremity fracture eponyms, focusing on imaging features to identify and differentiate these injuries. We focus on plain radiographic findings, with supporting computed tomography (CT) images. For each injury, important radiologic descriptors are discussed which may need to be communicated to clinicians. Aspects of management and follow-up imaging recommendations are included. This is a two-part review: Part 1 focuses on fracture eponyms of the upper extremity, while Part 2 encompasses fracture eponyms of the lower extremity. Keywords: Eponyms; Fractures; Lower extremities; Imaging Introduction Review: Lower extremity fracture eponyms Eponyms are embedded throughout medicine; they Pipkin fracture can be found in medical literature, textbooks, and Femoral head fractures are relatively uncommon and even mass media. Their use allows physicians to are typically associated with hip dislocations after se- quickly provide a concise description of a complex vere high-impact trauma such as a motor vehicle colli- injury pattern. Eponymous extremity fractures are sion. Femoral head fractures are commonly grouped commonly encountered in the emergency setting and into the Pipkin classification (see Table 1) after the work are frequently used in interactions amongst radiolo- of the orthopedic surgeon Garrett Pipkin in 1957 (Fig. -
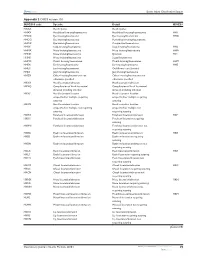
Appendix 2 OSICS Version 10.1 (Continued)
Dovepress Sports Injury Classification System Appendix 2 OSICS version 10.1 OSICS10 code Specific Detail OSICS9 HXXX Head injuries Head injuries HHXX Head/facial bruising/haematoma Head/facial bruising/haematoma HH1 HHOX Eye bruising/haematoma Eye bruising/haematoma HHO HHOO Eye bruising/haematoma Periorbital bruising/haematoma HHOC Eye bruising/haematoma Conjunctival haematoma HHSX Scalp bruising/haematoma Scalp bruising/haematoma HHS HHNX Nose bruising/haematoma Nose bruising/haematoma HHN HHNE Nose bruising/haematoma Epistaxis HV1 HHNS Nose bruising/haematoma Septal haematoma HHMX Mouth bruising/haematoma Mouth bruising/haematoma HHM HHEX Ear bruising/haematoma Ear bruising/haematoma HHE HHEC Ear bruising/haematoma Cauliflower ear (chronic) HHJX Jaw bruising/haematoma Jaw bruising/haematoma HHZX Other bruising/haematoma not Other bruising/haematoma not otherwise specified otherwise specified HKXX Head laceration/abrasion Head laceration/abrasion HKXQ Complication of head laceration/ Complication of head laceration/ abrasion including infection abrasion including infection HKXS Head laceration location Head laceration location unspecified/or multiple requiring unspecified/or multiple requiring suturing suturing HKXN Head laceration location Head laceration location unspecified/or multiple not requiring unspecified/or multiple not suturing requiring suturing HKHX Forehead laceration/abrasion Forehead laceration/abrasion HKF HKHS Forehead laceration/abrasion Forehead laceration requiring suturing HKHN Forehead laceration/abrasion Forehead