(And Why) Fins Turn Into Limbs: Insights from Anglerfish
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Wingtips of the Pterosaurs: Anatomy, Aeronautical Function and Palaeogeography, Palaeoclimatology, Palaeoecology Xxx (2015) Xxx Xxx 3 Ecological Implications
Our reference: PALAEO 7445 P-authorquery-v11 AUTHOR QUERY FORM Journal: PALAEO Please e-mail your responses and any corrections to: Article Number: 7445 E-mail: [email protected] Dear Author, Please check your proof carefully and mark all corrections at the appropriate place in the proof (e.g., by using on-screen annotation in the PDF file) or compile them in a separate list. Note: if you opt to annotate the file with software other than Adobe Reader then please also highlight the appropriate place in the PDF file. To ensure fast publication of your paper please return your corrections within 48 hours. For correction or revision of any artwork, please consult http://www.elsevier.com/artworkinstructions. We were unable to process your file(s) fully electronically and have proceeded by Scanning (parts of) your Rekeying (parts of) your article Scanning the article artwork Any queries or remarks that have arisen during the processing of your manuscript are listed below and highlighted by flags in the proof. Click on the ‘Q’ link to go to the location in the proof. Location in article Query / Remark: click on the Q link to go Please insert your reply or correction at the corresponding line in the proof Q1 Your article is registered as a regular item and is being processed for inclusion in a regular issue of the journal. If this is NOT correct and your article belongs to a Special Issue/Collection please contact [email protected] immediately prior to returning your corrections. Q2 Please confirm that given names and surnames have been identified correctly. -

BONY FISHES 602 Bony Fishes
click for previous page BONY FISHES 602 Bony Fishes GENERAL REMARKS by K.E. Carpenter, Old Dominion University, Virginia, USA ony fishes constitute the bulk, by far, of both the diversity and total landings of marine organisms encoun- Btered in fisheries of the Western Central Atlantic.They are found in all macrofaunal marine and estuarine habitats and exhibit a lavish array of adaptations to these environments. This extreme diversity of form and taxa presents an exceptional challenge for identification. There are 30 orders and 269 families of bony fishes presented in this guide, representing all families known from the area. Each order and family presents a unique suite of taxonomic problems and relevant characters. The purpose of this preliminary section on technical terms and guide to orders and families is to serve as an introduction and initial identification guide to this taxonomic diversity. It should also serve as a general reference for those features most commonly used in identification of bony fishes throughout the remaining volumes. However, I cannot begin to introduce the many facets of fish biology relevant to understanding the diversity of fishes in a few pages. For this, the reader is directed to one of the several general texts on fish biology such as the ones by Bond (1996), Moyle and Cech (1996), and Helfman et al.(1997) listed below. A general introduction to the fisheries of bony fishes in this region is given in the introduction to these volumes. Taxonomic details relevant to a specific family are explained under each of the appropriate family sections. The classification of bony fishes continues to transform as our knowledge of their evolutionary relationships improves. -

Your Inner Fish
CHAPTER THREE HANDY GENES While my colleagues and I were digging up the first Tiktaalik in the Arctic in July 2004, Randy Dahn, a researcher in my laboratory, was sweating it out on the South Side of Chicago doing genetic experiments on the embryos of sharks and skates, cousins of stingrays. You’ve probably seen small black egg cases, known as mermaid’s purses, on the beach. Inside the purse once lay an egg with yolk, which developed into an embryonic skate or ray. Over the years, Randy has spent hundreds of hours experimenting with the embryos inside these egg cases, often working well past midnight. During the fateful summer of 2004, Randy was taking these cases and injecting a molecular version of vitamin A into the eggs. After that he would let the eggs develop for several months until they hatched. His experiments may seem to be a bizarre way to spend the better part of a year, let alone for a young scientist to launch a promising scientific career. Why sharks? Why a form of vitamin A? 61 To make sense of these experiments, we need to step back and look at what we hope they might explain. What we are really getting at in this chapter is the recipe, written in our DNA, that builds our bodies from a single egg. When sperm fertilizes an egg, that fertilized egg does not contain a tiny hand, for instance. The hand is built from the information contained in that single cell. This takes us to a very profound problem. -

Phylogeny of a Rapidly Evolving Clade: the Cichlid Fishes of Lake Malawi
Proc. Natl. Acad. Sci. USA Vol. 96, pp. 5107–5110, April 1999 Evolution Phylogeny of a rapidly evolving clade: The cichlid fishes of Lake Malawi, East Africa (adaptive radiationysexual selectionyspeciationyamplified fragment length polymorphismylineage sorting) R. C. ALBERTSON,J.A.MARKERT,P.D.DANLEY, AND T. D. KOCHER† Department of Zoology and Program in Genetics, University of New Hampshire, Durham, NH 03824 Communicated by John C. Avise, University of Georgia, Athens, GA, March 12, 1999 (received for review December 17, 1998) ABSTRACT Lake Malawi contains a flock of >500 spe- sponsible for speciation, then we expect that sister taxa will cies of cichlid fish that have evolved from a common ancestor frequently differ in color pattern but not morphology. within the last million years. The rapid diversification of this Most attempts to determine the relationships among cichlid group has been attributed to morphological adaptation and to species have used morphological characters, which may be sexual selection, but the relative timing and importance of prone to convergence (8). Molecular sequences normally these mechanisms is not known. A phylogeny of the group provide the independent estimate of phylogeny needed to infer would help identify the role each mechanism has played in the evolutionary mechanisms. The Lake Malawi cichlids, however, evolution of the flock. Previous attempts to reconstruct the are speciating faster than alleles can become fixed within a relationships among these taxa using molecular methods have species (9, 10). The coalescence of mtDNA haplotypes found been frustrated by the persistence of ancestral polymorphisms within populations predates the origin of many species (11). In within species. -

Median Fin Patterning in Bony Fish: Caspase-3 Role in Fin Fold Reabsorption
Eastern Illinois University The Keep Undergraduate Honors Theses Honors College 2017 Median Fin Patterning in Bony Fish: Caspase-3 Role in Fin Fold Reabsorption Kaitlyn Ann Hammock Follow this and additional works at: https://thekeep.eiu.edu/honors_theses Part of the Animal Sciences Commons Median fin patterning in bony fish: caspase-3 role in fin fold reabsorption BY Kaitlyn Ann Hammock UNDERGRADUATE THESIS Submitted in partial fulfillment of the requirement for obtaining UNDERGRADUATE DEPARTMENTAL HONORS Department of Biological Sciences along with the HonorsCollege at EASTERN ILLINOIS UNIVERSITY Charleston, Illinois 2017 I hereby recommend this thesis to be accepted as fulfilling the thesis requirement for obtaining Undergraduate Departmental Honors Date '.fHESIS ADVI 1 Date HONORSCOORDmATOR f C I//' ' / ·12 1' J Date, , DEPARTME TCHAIR Abstract Fish larvae develop a fin fold that will later be replaced by the median fins. I hypothesize that finfold reabsorption is part of the initial patterning of the median fins,and that caspase-3, an apoptosis marker, will be expressed in the fin fold during reabsorption. I analyzed time series of larvae in the first20-days post hatch (dph) to determine timing of median findevelopment in a basal bony fish- sturgeon- and in zebrafish, a derived bony fish. I am expecting the general activation pathway to be conserved in both fishesbut, the timing and location of cell death to differ.The dorsal fin foldis the firstto be reabsorbed in the sturgeon starting at 2 dph and rays formed at 6dph. This was closely followed by the anal finat 3 dph, rays at 9 dph and only later, at 6dph, does the caudal fin start forming and rays at 14 dph. -

Pterosaurs Flight in the Age of Dinosaurs Now Open 2 News at the Museum 3
Member Magazine Spring 2014 Vol. 39 No. 2 Pterosaurs Flight in the Age of Dinosaurs now open 2 News at the Museum 3 From the After an unseasonably cold, snowy winter, will work to identify items from your collection, More than 540,000 Marine Fossils the Museum is pleased to offer a number of while also displaying intriguing specimens from President springtime opportunities to awaken the inner the Museum’s own world-renowned collections. Added to Paleontology Collection naturalist in us all. This is the time of year when Of course, fieldwork and collecting have Ellen V. Futter Museum scientists prepare for the summer been hallmarks of the Museum’s work since Collections at a Glance field season as they continue to pursue new the institution’s founding. What has changed, discoveries in their fields. It’s also when Museum however, is technology. With a nod to the many Over nearly 150 years of acquisitions and Members and visitors can learn about their ways that technology is amplifying how scientific fieldwork, the Museum has amassed preeminent own discoveries during the annual Identification investigations are done, this year, ID Day visitors collections that form an irreplaceable record Day in Theodore Roosevelt Memorial Hall. can learn how scientists use digital fabrication of life on Earth. Today, 21st-century tools— Held this year on May 10, Identification Day to aid their research and have a chance to sophisticated imaging techniques, genomic invites visitors to bring their own backyard finds have their own objects scanned and printed on analyses, programs to analyze ever-growing and curios for identification by Museum scientists. -

Blackchin Tilapia (Sarotherodon Melanotheron) Ecological Risk Screening Summary
U.S. Fish and Wildlife Service Blackchin Tilapia (Sarotherodon melanotheron) Ecological Risk Screening Summary Web Version – 10/01/2012 Photo: © U.S. Geological Survey From Nico and Neilson (2014). 1 Native Range and Nonindigenous Occurrences Native Range From Nico and Neilson (2014): “Tropical Africa. Brackish estuaries and lagoons from Senegal to Zaire (Trewavas 1983).” Nonindigenous Occurrences From Nico and Neilson (2014): “Established in Florida and Hawaii. Evidence indicates it is spreading rapidly in both fresh and salt water around island of Oahu, Hawaii (Devick 1991b).” “The first documented occurrence of this species in Florida was a specimen gillnetted by commercial fishermen in Hillsborough Bay near Tampa, Hillsborough County, in 1959 (Springer and Finucane 1963). Additional records for the western part of the state indicate that this species is established in brackish and freshwaters in eastern Tampa Bay and in adjoining drainages in Hillsborough County, ranging from the Alafia River south to Cockroach Bay. The species has been recorded from the Alafia River from its mouth up to Lithia Springs; from the Hillsborough River, Bullfrog Creek, the Palm River, and the Little Manatee River; and from various western drainage and irrigation ditches (Springer and Finucane 1963; Finucane and Rinckey 1967; Buntz Sarotherodon melanotheron Ecological Risk Screening Summary U.S. Fish and Wildlife Service – Web Version – 10/01/2012 and Manooch 1969; Lachner et al. 1970; Courtenay et al. 1974; Courtenay and Hensley 1979; Courtenay and Kohler 1986; Lee et al. 1980 et seq.; Courtenay and Stauffer 1990; DNR collections; UF museum specimens). There are two records of this species from the west side of Tampa Bay, in Pinellas County: a collection from Lake Maggiore in St. -

Human Functional Anatomy 213 the Upper Limb Early Limb Development
2 HUMAN FUNCTIONAL ANATOMY 213 THE UPPER LIMB EARLY LIMB DEVELOPMENT THIS WEEKS LAB: IN THE FISH (or early human embryo) Proximal parts, plexuses and patterns Slight elevations of ectoderm appear in lateral plate (4th week). Apical ectodermal ridge induces proliferation of limb mesenchyme. READINGS Dorsal and ventral muscle masses connect the girdle to the limb bud. Stern. Essentials of Gross anatomy – The upper limb Limb girdle in body wall Stern. Core concepts in Anatomy:- 80: Organization of upper limb musculature and the Proximal, middle and distal segments of the limb brachial plexus Faiz and Moffat. Anatomy at a Glance:- Nerves of the Upper limb 1 & 2 (parts 30 &31) Grant's Method:- Upper limb and Back (especially pectoral region and axilla) OR any other regional textbook - similar sections IN THIS LECTURE I WILL COVER: Ontogeny and Phylogeny The Pectoral fin The primitive tetrapod forelimb Rotations of the limb in phylogeny Dorsal and ventral muscle/nerve/girdle bone Segmental nerve supply and muscle groups Brachial plexus Muscle groups of the upper limb The fin, or paddle has: Preaxial and postaxial borders (front and back edges) Dorsal and ventral surfaces (top and bottom) Dorsal muscles elevate the fin. Attach to dorsal elements of the girdle (“scapula” and vertebrae) Ventral muscles depress the fin. Attach to ventral elements of the girdle (coracoid) 3 4 PRIMITIVE TETRAPOD FORELIMB MAMMALIAN FORELIMB ROTATIONS 90 degrees LATERAL ROTATION The characteristic segments of the limb (shoulder, arm, forearm, & hand) Were present in -

Southampton French Quarter 1382 Specialist Report Download E2: Fish Bone
Southampton French Quarter SOU1382 Specialist Report Download E2 Southampton French Quarter 1382 Specialist Report Download E2: Fish Bone By Rebecca Nicholson Introduction An assemblage of almost 7500 identifiable fish bones was recovered both by hand retrieval during the excavation, but predominantly from the sorted residues of the processed bulk soil samples. During excavations at Southampton French Quarter a total of 188 bulk samples were sieved to 0.5mm (occasionally 1mm) as part of the flotation process for the recovery of plant and animal remains. The sampling strategy followed during the excavation involved, where possible, the full sampling of one rubbish pit and one latrine pit per tenement for each major period, avoiding intercut features or those clearly containing residual material. Occupation surfaces and other distinct features such as hearths were also sampled. Mixed contexts or contexts of uncertain provenance were avoided. While complete standardisation of sample volumes was not possible, wherever practicable samples were 40 litres. Following assessment of the fish remains recovered largely from the > 4mm residues, the richer assemblages were targeted for further fine residue and flot sorting. This report comprises an analysis of all the identified the fish remains from these samples, together with the material collected by hand on site. Methodology The residues from all of the bulk-sieved samples were sorted to 4mm. Where samples were identified as having significant numbers of fish bones, residues were sorted to 2mm. Samples from the Late Saxon deposits which produced fish remains were routinely sorted to 2mm even where fish remains were not abundant, in order to avoid a perceived bias against the recovery of small fish in pre-medieval deposits (see Barrett et al. -

PEIXES RECIFAIS DO NORDESTE BRASILEIRO.Pdf
UNIVERSIDADE FEDERAL DA PARAÍBA CENTRO DE CIÊNCIAS EXATAS E DA NATUREZA CURSO DE BACHARELADO EM CIÊNCIAS BIOLÓGICAS DIVERSIDADE DOS PEIXES RECIFAIS DO NORDESTE BRASILEIRO Jessé Miranda de Figueiredo Filho Ricardo de Souza Rosa Orientador João Pessoa – 2016 UNIVERSIDADE FEDERAL DA PARAÍBA CENTRO DE CIÊNCIAS EXATAS E DA NATUREZA CURSO DE BACHARELADO EM CIÊNCIAS BIOLÓGICAS DIVERSIDADE DOS PEIXES RECIFAIS DO NORDESTE BRASILEIRO Jessé Miranda de Figueiredo Filho Ricardo de Souza Rosa Orientador Monografia apresentada ao Curso de Ciências Biológicas como requisito parcial à obtenção do grau de Bacharel em Ciências Biológicas. João Pessoa – 2016 Catalogação na publicação Universidade Federal da Paraíba Biblioteca Setorial do CCEN Maria Teresa Macau - CRB 15/176 F475d Figueiredo Filho, Jessé Miranda de. Diversidade dos peixes recifais do Nordeste Brasileiro / Jessé Miranda de Figueiredo Filho. – João Pessoa, 2016. 40p. : il.- Monografia ( Bacharelado em Ciências Biológicas ) – Universidade Federal da Paraíba. Orientador: Profº Drº Ricardo de Souza Rosa. 1. Peixes. 2. Recifes. 3. Lista sistemática. I. Título. CDU : 597.2/.5(043.2) “By the deep sea, and music in its roar I love not man the less, but nature more.” (George Gordon Byron) AGRADECIMENTOS À minha família – em especial aos meus pais, Jessé Figueiredo e Tânia Ribeiro, por todo o apoio, confiança, carinho e conselhos que depositaram em mim durante toda a minha vida, pela ajuda nos momentos difíceis e comemorações nas alegrias da vida, sem vocês eu não teria conseguido. À minha irmã, Tayná Ribeiro, pelo apoio, sinceridade, discussões, e por sempre acreditar em mim. Ao meu orientador, Dr. Ricardo Rosa, pela orientação, paciência e confiança; por ter me apresentado ao mundo fantástico da ictiologia, e acreditado no meu potencial. -
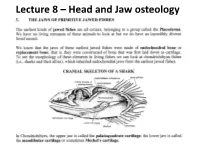
Lecture 8 – Head and Jaw Osteology
Lecture 8 – Head and Jaw osteology More derived fishes (Ray finned fishes) The variability of the jaw structure of bony fishes provides an explanation for the extensive adaptive radiation in the group and why they are so diverse and occupy almost every aquatic niche available. Skull diversity (A) carp, Cyprinus carpio, (B) vampire characin, Hydrolycus scomberoides, (C) catfish Arius felis. (D) cod Gadus morhua. (E) large-mouth bass, Micropterus salmoides (F) The parrotfish Scarus guacamaia. Scale bar = 10 mm WESTNEAT 2004 From an evolutionary standpoint, fishes were the first animals to develop bony jaws. Versatile jaws and multiple feeding strategies allowed fishes to fill, or radiate into, a diverse range of niches. They have evolved to feed in all possible ways – sucking, biting, scraping, nipping, crushing etc. The head of a teleost has 5 main regions: Cranium, jaws, cheeks, hydroid arch, opercula. The head of a fish has five main regions • 1) The CRANIUM is composed of the bones providing direct support and protection to the brain and the visual, Anterior Posterior olfactory, and auditory organs. Below the cranium is the parashenoid bone. • Parasphenoid plays a role in the jaws as Features of the neurocranium sensu lato (from Caranx it acts as a hard melampygus, lateral aspect, left, and posterior aspect, right). A = prevomer, B = ethmoid, C = frontal, D = palate supraoccipital, E = pterotic, F = exoccipital, G = basioccipital, H = foramen magnum, I = parasphenoid, J = orbit. The five main regions Bowfin 2) The JAWS • Lower Jaw – has an Angular articular and dentary bone • Angular articular- The paired bones form the posterior part of either side of the lower jaw and articulate with the suspensorium. -

Biology of Chordates Video Guide
Branches on the Tree of Life DVD – CHORDATES Written and photographed by David Denning and Bruce Russell ©2005, BioMEDIA ASSOCIATES (THUMBNAIL IMAGES IN THIS GUIDE ARE FROM THE DVD PROGRAM) .. .. To many students, the phylum Chordata doesn’t seem to make much sense. It contains such apparently disparate animals as tunicates (sea squirts), lancelets, fish and humans. This program explores the evolution, structure and classification of chordates with the main goal to clarify the unity of Phylum Chordata. All chordates possess four characteristics that define the phylum, although in most species, these characteristics can only be seen during a relatively small portion of the life cycle (and this is often an embryonic or larval stage, when the animal is difficult to observe). These defining characteristics are: the notochord (dorsal stiffening rod), a hollow dorsal nerve cord; pharyngeal gills; and a post anal tail that includes the notochord and nerve cord. Subphylum Urochordata The most primitive chordates are the tunicates or sea squirts, and closely related groups such as the larvaceans (Appendicularians). In tunicates, the chordate characteristics can be observed only by examining the entire life cycle. The adult feeds using a ‘pharyngeal basket’, a type of pharyngeal gill formed into a mesh-like basket. Cilia on the gill draw water into the mouth, through the basket mesh and out the excurrent siphon. Tunicates have an unusual heart which pumps by ‘wringing out’. It also reverses direction periodically. Tunicates are usually hermaphroditic, often casting eggs and sperm directly into the sea. After fertilization, the zygote develops into a ‘tadpole larva’. This swimming larva shows the remaining three chordate characters - notochord, dorsal nerve cord and post-anal tail.