Modelling and Surgical Analysis of Fracture Repair of the Facial Skeleton Using Generic and Patient Matched Finite Element Models
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mandibular Fractures, Diagnostics, Postoperative Complications
Journal of Medical Sciences. March 23, 2020 - Volume 8 | Issue 13. Electronic-ISSN: 2345-0592 Medical Sciences 2020 Vol. 8 (13), p. 45-52 e-ISSN: 2345-0592 Medical Sciences Online issue Indexed in Index Copernicus Official website: www.medicsciences.com Mandibular fractures, diagnostics, postoperative complications Shahaf Givony1 1 Lithuanian University of Health Sciences. Academy of Medicine. Faculty of Odonthology. ABSTRACT Mandibular fractures usually happen among young males at the age of 16-30 years old. The mandible which has been rated as the second facial bone with the highest rate of injuries, tends to break much more often compared to any other bone of the cranium and represent up to 70% of the cases. This tendency to fracture may be explained by the protruded position, mobility and particular shape of it. The tendency for a mandibular fracture may also be explained by the common risk factors such as vehicle accidents and physical violence that are part of our daily life. There are many other risk factors according to the literature which differ between individuals due to the different socio-economic status, culture, technology and environment. Before the clinical examination of the fracture, it is obligatory to make sure that a clear airway path presents with no other fatal injuries. The examination may be supported by imaging methods which together will approve the diagnosis and method of treatment. Patients with a fracture of the mandible may suffer from post-operative complications which may occur after a short or long duration of the treatment. Those complications may be malocclusion, infections, trismus, damaged teeth and soft tissue, esthetic disfiguration, functional problems, pain and many more. -

Pattern of Skeletal Injuries in Child Physical Abuse
1 Bahrain Medical Bulletin, Vol. 33, No. 2, June 2011 Pattern of Skeletal Injuries in Physically Abused Children Fadheela Al-Mahroos, MD, MHPE* Eshraq A Al-Amer, MD, ABMS (Ped)** Nabar J Umesh, MD, DMRE*** Ali I Alekri, FRCSI, CABS (Ortho)**** Objective: The aim of this study is to identify the frequency and patterns of skeletal injuries among victims of child abuse in Bahrain. Design: Retrospective. Setting: Child Protection Unit at Salmaniya Medical Complex. Method: Child’s characteristics, type of skeletal injuries, location, pattern, radiological findings, and associated other injuries of 36 children were reviewed. Data management and analysis was done using SPSS for Windows, version 18. Result: Thirty-six children with skeletal injuries resulting from child physical abuse were seen from 1991 to 2009. Twenty-three (64%) were males and 13 (36%) were females; the mean age was 3.8 years. Twenty-three (64%) were ≤ 3 years old. Multiple fractures were documented in 19 (53%) children. Bone fracture types and frequency were as follow: 10 (28%) affecting the femur, 9 (25%) skull, 8 (22%) humerus, 6 (17%) rib, 4 (11%) radius, 4 (11%) ulna and 2 (6%) tibia. Other bones less frequently affected were mandible, nasal bone, vertebral, metatarsals, and calcaneus fractures. In addition, other injuries included slipped femoral epiphysis, large bilateral hematoma in vastus lateralis, and full thickness tendon Achilles tear. Hundred percent of rib, ulnar, radial and tibial fractures were in children under one year old. In addition, 7 (78%) of skull fractures, 5 (62%) of humerus fractures, and 5 (50) of femur fractures were under one year old. -

Anatomy Review Upper Extremity WARNING: the Content in This Slideshow Contains Some Sports Footage That May Be Gross
BONES Anatomy Review Upper Extremity WARNING: The content in this slideshow contains some sports footage that may be gross. If you don’t like bodily injuries be warned! SKULL/ FACE Four major bones fuse together to create our cranium . → parietal,temporal,occipital and frontal bones Two major bones of the jaw . → Mandible and Maxilla INJURIES TO THE SKULL & FACE This is raccoon eyes, and battle sign which indicate a skull fracture. Common cause is blunt force trauma to the head, for example, getting hit in the head with a baseball. Often seen in individuals who are beaten or abused. INJURIES TO THE SKULL & FACE This is a picture of Victor Hedman a top defenseman in the NHL. INJURY: laceration to the face. CAUSE: helmet visor was pushed into his face. Lacerations are common during sports and either treated with stitches or glue INJURIES TO THE SKULL & FACE Mandibular Fracture Video Link above is for a Fracture to the mandible Sidney Crosby was hit in the face by a 90 mph slapshot! INJURY: Mandibular Fracture TREATMENT: Surgery ( Jaw was wired shut) He was also provided with a Jaw shield ANTERIOR TORSO Ribs- Remember ribs are discussed in pairs True ribs vs. False ribs- true ribs connect directly to the sternum false ribs do not. Sternum- Broken down into three parts: 1. Manubrium 2. Body 3. Xiphoid process INJURIES TO THE ANTERIOR TORSO INJURY: Fractured Ribs CAUSE: A direct blow or trauma to the ribs Usually detected on x-ray POSTERIOR TORSO Vertebrae → 7 cervical 12 thoracic 5 lumbar BONES OF THE ARM 3 Major bones make up the shoulder: 1. -

Coleman Sign: a Hallmark for Mandibular Fracture? a Rare Case Exception
European Journal of Molecular & Clinical Medicine ISSN 2515-8260 Volume 07, Issue 10, 2020 Coleman Sign: A Hallmark For Mandibular Fracture? A Rare Case Exception Dr Premalatha Shetty(MDS)1, Dr Aditya Nandan, 2, Dr Mahabalesh Shetty3, Dr Suraj Shetty4 1Associate Dean, Professor, Oral and Maxillofacial department, Manipal College of Dental sciences, Mangalore, Manipal Academy of Higher Education, Manipal 2 Postgraduate(BDS), Oral and Maxillofacial department, Manipal College of dental sciences, Mangalore, Manipal Academy of Higher Education, Manipal 3Professor& Head of Department, Department of Forensic Medicine & Toxicology K S Hegde Medical Academy, NIITE (Deemed to be University) 4Associate Professor, Department of Forensic Medicine & Toxicology, K S Hegde Medical Academy, NIITE (Deemed to be University) Email ID:[email protected], [email protected], [email protected], [email protected] Abstract: Mandibular fracture involving the parasymphysis region is one of the most common fractures of mandible. Majority of parasymphysis fractures are due to direct blow or injury to the chin region. Specific signs and symptoms for parasymphysis fracture are pain, swelling, tenderness in the chin region, deranged occlusion, soft tissue injury to chin and the lower lip and sublingual hematoma. Frank Coleman considered a sublingual hematoma as “almost pathognomonic of fracture of the mandible”. We present a case that fails to replicate this hallmark sign associated with a mandibular fracture, as the patient had all the signs and symptoms of parasymphysis fracture except sublingual hematoma which is very rare and unusual to observe. The final diagnosis was made on the basis of radiographic examination using CBCT scan. On surgical exposure the mandibular parasymphysis fracture in the region of right lateral incisor and canine was confirmed. -

Imaging of Mandibular Fractures: a Pictorial Review
Nardi et al. Insights into Imaging (2020) 11:30 https://doi.org/10.1186/s13244-020-0837-0 Insights into Imaging EDUCATIONAL REVIEW Open Access Imaging of mandibular fractures: a pictorial review Cosimo Nardi1, Chiara Vignoli1, Michele Pietragalla1, Paolina Tonelli1, Linda Calistri1, Lorenzo Franchi2,3, Lorenzo Preda4,5* and Stefano Colagrande1 Abstract Mandibular fractures are among the most common maxillofacial fractures observed in emergency rooms and are mainly caused by road accidents. The clinical features of mandibular fractures include malocclusion and loss of mandibular function. Panoramic radiography is usually limited to isolated lesions, whereas computed tomography is the tool of choice for all other facial traumatic events. No reference standard classification system for the different types of mandibular fractures is defined. Therapeutic options include a conservative approach or surgical treatment based on the anatomic area and the severity of fracture. The main purpose of this pictorial review is to illustrate a practical description of the pathophysiology of mandibular fractures and describe both the imaging techniques to recognise them and the therapeutic indications. Keywords: Mandible, Condyle, Fracture, Trauma, Panoramic radiography Key points maxillofacial fractures varies according to geographical Mandibular fractures represent two thirds of all areas and socio-economic factors. The most common maxillofacial fractures. causes of maxillofacial fractures are road traffic accidents X-ray films, including panoramic radiography, are (40–42%), falls, assaults, sports, and work injuries [3]. usually limited to mild traumatic events. The average age of patients with mandibular fracture is Computed tomography is the tool of choice for the 38 years for men and 40 years for women [4]. -

Delayed Post Traumatic Facial Nerve Palsy on Contra Lateral Side of Isolated Mandibular Fracture: a Rare Case Report
International Journal of Dental and Health Sciences Case Report Volume 01,Issue 01 DELAYED POST TRAUMATIC FACIAL NERVE PALSY ON CONTRA LATERAL SIDE OF ISOLATED MANDIBULAR FRACTURE: A RARE CASE REPORT Monika Khangwal1, Ravinder Solanki2, Amit Bali3, Yashanshi Khanna4, Davender Kumar5, Rajiv Tanwar6 1.Post Graduate Student,Department Of Endodontics, King George Medical University, Lucknow. 2.Assistant Professor, Department Of Facio-Maxillary Surgery, Post Graduate Institute Of Dental Sciences, University Of Health Sciences, Rohtak ,Haryana. 3.Reader, MM Dental College, Mullana, Haryana 4.BDS Student, Sudha Rustogi Dental College, Faridabad, Haryana. 5.Assistant Professor,Post Graduate Institute Of Dental Sciences, Rohtak Haryana. 6.Post Graduate Student, Oral & Maxillofacial Surgery,Post Graduate Institute Of Dental Sciences,Rohtak,Haryana. ABSTRACT Post traumatic facial nerve paralysis is not a common complication. Only 5% of all the facial palsies are due to trauma. Moreover, it is common due to fracture of the temporal bone with transaction or compression of the facial nerve. Less commonly facial paralysis due to isolated mandibular fracture without temporal bone fracture have been reported in literature. Rarely, mandibular fractures can present with concomitant facial nerve injury. In cases where the nerve injury is incomplete or delayed, prognosis is excellent. This article reports a case of delayed onset of facial paralysis with isolated mandibular fracture. Key words: Delayed, Bell's palsy, Mandibular fracture, Aetiology, Trauma INTRODUCTION: agreement on definition, there is no consensus regarding the aetiology, Idiopathic peripheral facial nerve paralysis diagnostic approach, or management of is the most common cause of facial nerve this condition .[2] The aetiology of the palsy. -

Complications of Mandibular Fracture Repair and Secondary Reconstruction
225 Complications of Mandibular Fracture Repair and Secondary Reconstruction Daniel Perez, DDS1 Edward Ellis III, DDS1 1 Department of Oral and Maxillofacial Surgery, University of Texas Address for correspondence Edward Ellis III, DDS, Department of Oral Health Science Center at San Antonio, San Antonio, Texas and Maxillofacial Surgery, University of Texas Health Science Center at San Antonio, 8210 Floyd Curl Dr. MC8124, San Antonio, Semin Plast Surg 2020;34:225–231. TX 78229-3923 (e-mail: [email protected]). Abstract Mandibular fractures are common facial injuries. Their treatment varies as do Keywords postoperative complications. This paper discusses the common complications that ► complications are associated with the treatment of mandibular fractures and presents management ► mandible strategies. ► fractures The most common complication cited in the literature after typically would spare CN VII but might affect CN V. Some treatment of a mandibular fracture is infection followed closely areas are more difficult to access and repair, which, in turn, by malunion.1 Many surgeons agree that preoperative infec- increases surgical time, infection rates, and improper appli- tion, greater displacement of the fragments, and the presence cation of plates and screws. of multiple fractures and comorbidities increase the risk of – complications.1 4 Hardware-Related Complications The purpose of this article is to present and discuss the Here we can find material wear and fatigue leading to most common complications a surgeon can face when treat- material breakage, hypersensitivity, and allergic reactions, ing mandibular fractures and to delineate their causes and as well as improper selection or application of fixation. management. A corollary is to provide guidance to decrease the incidence of complications. -

Treatment of Mandible Fractures Using a Miniplate System: a Retrospective Analysis
Journal of Clinical Medicine Article Treatment of Mandible Fractures Using a Miniplate System: A Retrospective Analysis 1, , 1, 1 2 Lauren Bohner * y , Fabian Beiglböck y, Stephanie Schwipper ,Rômulo Maciel Lustosa , Carla Pieirna Marino Segura 3, Johannes Kleinheinz 1 and Susanne Jung 1 1 Department of Oral and Maxillofacial Surgery, University Hospital Münster, 48149 Münster, Germany; [email protected] (F.B); [email protected] (S.S.); [email protected] (J.K.); [email protected] (S.J.) 2 Department of Dentistry, State University of Maringá, 87020-900 Maringá, Brazil; [email protected] 3 Department of Head and Neck Surgery Daniel Alcides Carrion National Hospital, 07016 Bellavista, Peru; [email protected] * Correspondence: [email protected]; Tel.: +49-25183-47004 L.B. and F.B. shared the first author position. y Received: 18 August 2020; Accepted: 8 September 2020; Published: 10 September 2020 Abstract: Three-dimensional (3D) mini plate systems are used in the treatment of mandibular fractures. The system is advantageous in comparison to conventional plates due to the stabilization of tension and compression areas, improved initial stability, and biomechanical behavior. The aim of this retrospective study was to evaluate the use of a 3D miniplate system for the treatment of patients with mandibular fractures. Patients with mandibular fractures treated with a 3D plate system at the Department of Oral and Maxillofacial Surgery, University Hospital Münster, during a period of 5 years, were included in this study. Mandibular fracture conditions and minor and major post-operative complications were reported. In total, 336 patients and 391 mandibular fractures were assessed. -

Mandibular Fracture in a Patient Treated with Long-Term
Case Report Mandibular fracture in a patient treated with long-term antiangiogenic therapy and previous bisphosphonate exposure Tyler Davis, MD,1 Don-John Summerlin, MD,2 Michael Moore, MD,3 Anne Younger, RN,4 and E. Gabriela Chiorean, MD1,4 Departments of 1 Medicine, 2 Pathology, and 3 Otolaryngology, 4 Melvin and Bren Simon Cancer Center, Indiana University, Indianapolis, IN isphosponate-related osteonecrosis a mandibular fracture while he was off zoledronic of the jaw (ONJ) has been reported acid for 15 months but undergoing treatment with in the literature since 2003,1,2 with paclitaxel and bevacizumab plus sorafenib on a more than 90% of the events attribut- clinical trial. The case may help explain the tempo- ed to treatment with bisphosphonate ral relationship between therapy and the occurrence agents, such as zoledronic acid (Zometa) and pami- of jaw fracture, as well as the link between ONJ and dronate, which are used to treat hypercalcemia of the risk of fractures with sequential use of bisphos- malignancy and to reduce the risk of skeletal-related phonates and antiangiogenic agents. B 3 –5 events due to bone metastases. Patients with ONJ Case presentation generally present with exposed necrotic bone that does not heal for 6–8 weeks, often leading to signifi- A 65-year-old man was diagnosed with stage cant morbidity. The pathogenic mechanism for os- IIB (T2cN0M0) prostate cancer and initially teonecrosis is unclear, but, in addition to inhibiting treated with prostatectomy in 1997. He devel- bone resorption, preclinical data have demonstrated oped a biochemical recurrence, with a rise in pros- thrombotic microangiopathy and potent inhibition tate-specific antigen (PSA) level, in 2000 and was of angiogenesis,6 which may lead to avascular necro- treated with local radiotherapy followed by bi- sis and poor wound healing after dental procedures. -

Complex Rehabilitation of Patients with Jaw Fractures
www.symbiosisonline.org Symbiosis www.symbiosisonlinepublishing.com Review article Journal of Dentistry, Oral Disorders & Therapy Open Access Complex Rehabilitation of Patients with Jaw Fractures Bobamuratova DT1* and Boymuradov SHA2 1PhD Candidate (researcher), Department of Dentistry, Tashkent Medical Academy, Uzbekistan 2Doctor of medical sciences, Department of Otolaryngology and Dentistry, Department of the head of the the maxillofacial surgery, Tashkent Medical Academy, Uzbekistan Received: July 23, 2018; Accepted: August 08, 2018; Published: August 24, 2018 *Corresponding author: Bobamuratova DT, PhD Candidate (researcher), Department of Dentistry, Tashkent Medical Academy, Uzbekistan, 100109, Tel: +99897 7105911; E-mail: [email protected] disorders of the body’s functions. Rehabilitation is a part of Abstract daily care and reduces the consequences of the disease [39]. The This review article provides brief information on rehabilitation measures that improve the local and general condition of patients with injuries of the maxillofacial area is a multidisciplinary scientific substantiation of the rehabilitation system for patients with jaw fractures. problem and is an urgent task of modern medicine due to large economic losses associated with their high prevalence among In the recent year the number of injuries with maxillofacial persons of the most able-bodied age [7,22,32,33,50]. In recent maxillofacial injuries, fractures of the jaws occupy the main place (65- years, the world has been growing the tendency to increase 80%),trauma and has significantlymost of the increasedpatients are in all young countries and able-bodiedof the world. people.Among the chronic disability of patients with facial fractures and their Despite the continuous improvement of the treatment methods of rejuvenation. -

Clinical Outcome of Conservative Treatment of Displaced Mandibular Fracture in Adults
Surgical Science, 2013, 4, 500-505 Published Online November 2013 (http://www.scirp.org/journal/ss) http://dx.doi.org/10.4236/ss.2013.411097 Clinical Outcome of Conservative Treatment of Displaced Mandibular Fracture in Adults Lipa Bodner1*, Sigal Amitay1, Ben Zion Joshua2 1Department of Oral and Maxillofacial Surgery, Soroka Medical Center, Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer-Sheva, Israel 2Department of Otolaryngology Head and Neck Surgery, Soroka Medical Center, Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer-Sheva, Israel Email: *[email protected] Received September 3, 2013; revised October 1, 2013; accepted October 9, 2013 Copyright © 2013 Lipa Bodner et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT The article evaluates 12 cases of conservative treatment of displaced mandibular fractures in adults. Twelve cases of displaced mandibular fractures treated surgically, either by closed reduction (IMF) or open reduction internal fixation (ORIF) served as controls. Occlusion, maximal mouth opening, lateral jaw movements, neurological dysfunction (=sensory deficit), and bone remodeling were evaluated and scored in both groups, and results were compared. No sig- nificant differences were found between the two groups in all the evaluated parameters. It is concluded that in certain cases, with displacement of 2 - 4 mm, where a surgical approach is not feasible, reasonable spontaneous reduction and bone remodeling can occur. Meticulous follow-up is mandatory. Keywords: Mandible; Jaw Bone; Trauma; Displaced Fracture; Conservative Management 1. -
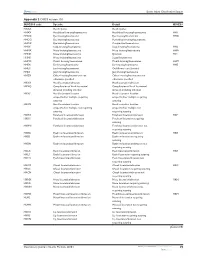
Appendix 2 OSICS Version 10.1 (Continued)
Dovepress Sports Injury Classification System Appendix 2 OSICS version 10.1 OSICS10 code Specific Detail OSICS9 HXXX Head injuries Head injuries HHXX Head/facial bruising/haematoma Head/facial bruising/haematoma HH1 HHOX Eye bruising/haematoma Eye bruising/haematoma HHO HHOO Eye bruising/haematoma Periorbital bruising/haematoma HHOC Eye bruising/haematoma Conjunctival haematoma HHSX Scalp bruising/haematoma Scalp bruising/haematoma HHS HHNX Nose bruising/haematoma Nose bruising/haematoma HHN HHNE Nose bruising/haematoma Epistaxis HV1 HHNS Nose bruising/haematoma Septal haematoma HHMX Mouth bruising/haematoma Mouth bruising/haematoma HHM HHEX Ear bruising/haematoma Ear bruising/haematoma HHE HHEC Ear bruising/haematoma Cauliflower ear (chronic) HHJX Jaw bruising/haematoma Jaw bruising/haematoma HHZX Other bruising/haematoma not Other bruising/haematoma not otherwise specified otherwise specified HKXX Head laceration/abrasion Head laceration/abrasion HKXQ Complication of head laceration/ Complication of head laceration/ abrasion including infection abrasion including infection HKXS Head laceration location Head laceration location unspecified/or multiple requiring unspecified/or multiple requiring suturing suturing HKXN Head laceration location Head laceration location unspecified/or multiple not requiring unspecified/or multiple not suturing requiring suturing HKHX Forehead laceration/abrasion Forehead laceration/abrasion HKF HKHS Forehead laceration/abrasion Forehead laceration requiring suturing HKHN Forehead laceration/abrasion Forehead