Changes in the Expression Pattern of Epidermal Stem Cell Markers In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Adherens Junctions, Desmosomes and Tight Junctions in Epidermal Barrier Function Johanna M
14 The Open Dermatology Journal, 2010, 4, 14-20 Open Access Adherens Junctions, Desmosomes and Tight Junctions in Epidermal Barrier Function Johanna M. Brandner1,§, Marek Haftek*,2,§ and Carien M. Niessen3,§ 1Department of Dermatology and Venerology, University Hospital Hamburg-Eppendorf, Hamburg, Germany 2University of Lyon, EA4169 Normal and Pathological Functions of Skin Barrier, E. Herriot Hospital, Lyon, France 3Department of Dermatology, Center for Molecular Medicine, Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD), University of Cologne, Germany Abstract: The skin is an indispensable barrier which protects the body from the uncontrolled loss of water and solutes as well as from chemical and physical assaults and the invasion of pathogens. In recent years several studies have suggested an important role of intercellular junctions for the barrier function of the epidermis. In this review we summarize our knowledge of the impact of adherens junctions, (corneo)-desmosomes and tight junctions on barrier function of the skin. Keywords: Cadherins, catenins, claudins, cell polarity, stratum corneum, skin diseases. INTRODUCTION ADHERENS JUNCTIONS The stratifying epidermis of the skin physically separates Adherens junctions are intercellular structures that couple the organism from its environment and serves as its first line intercellular adhesion to the cytoskeleton thereby creating a of structural and functional defense against dehydration, transcellular network that coordinate the behavior of a chemical substances, physical insults and micro-organisms. population of cells. Adherens junctions are dynamic entities The living cell layers of the epidermis are crucial in the and also function as signal platforms that regulate formation and maintenance of the barrier on two different cytoskeletal dynamics and cell polarity. -

Keratinization and Its Disorders
Oman Medical Journal (2012) Vol. 27, No. 5: 348-357 DOI 10. 5001/omj.2012.90 Review Article Keratinization and its Disorders Shibani Shetty, Gokul S. Received: 03 May 2012 / Accepted: 08 July 2012 © OMSB, 2012 Abstract Keratins are a diverse group of structural proteins that form the epithelium (buccal mucosa, labial mucosa) and specialized intermediate filament network responsible for maintaining the mucosa (dorsal surface of the tongue).2 An important aspect structural integrity of keratinocytes. In humans, there are around of stratified squamous epithelia is that the cells undergo a 30 keratin families divided into two groups, namely, acidic and terminal differentiation program that results in the formation basic keratins, which are arranged in pairs. They are expressed in of a mechanically resistant and toughened surface composed of a highly specific pattern related to the epithelial type and stage of cornified cells that are filled with keratin filaments and lack nuclei cellular differentiation. A total of 54 functional genes exist which and cytoplasmic organelles. In these squames, the cell membrane codes for these keratin families. The expression of specific keratin is replaced by a proteinaceous cornified envelope that is covalently genes is regulated by the differentiation of epithelial cells within cross linked to the keratin filaments, providing a highly insoluble the stratifying squamous epithelium. Mutations in most of these yet flexible structure that protects the underlying epithelial cells.1 genes are now associated with specific tissue fragility disorders Keratinization, also termed as cornification, is a process which may manifest both in skin and mucosa depending on the of cytodifferentiation which the keratinocytes undergo when expression pattern. -
Involucrin in Squamous and Basal Cell Carcinomas of the Skin: an Immunohistochemical Study
0022-202X/84/ 8205-0449$02.00/0 THE .JOURNAL OF I NVESTIGATI VE D E RMATOL OGY, 82:449- 452, 1984 Vol. 82 , No.5 Copyright © 1984 by The Wi lliams & Wilk ins Co. Printed in U.S.A. REPORTS Involucrin in Squamous and Basal Cell Carcinomas of the Skin: An Immunohistochemical Study JONATHAN W. SAID, M .D., AARON F. SASSOON, B.S., I. PETER SHINTAKU, PH.D., AND SusAN BANKS-SCHLEGEL, PH.D. Division of Anatomic Pathology, Cedars-Sinai Medical Center (JWS. IPS), Los Angeles, Califom.ia: Laboratory of Human Ca rcinogene~is, National Cancer Institute, National Institutes of Health (SB-SJ, Bethesda. Maryland; and Department of Biology. University of Southern California (AFS), Los Angeles, California, U.S. A. Involucrin is a precursor of the cross-linked envelope MATERIALS AND METHODS protein of human stratum corneum, and its appearance Cases of skin tumors (24 squamous cell and 21 basal cell carcinomas) in the upper layers of the epidermis is a function of the were retrieved from the files of the surgica l pathology division of normal differentiation of the keratinocyte. Cases of Cedars-Sinai Medical Center. In selected cases, sections were reviewed basal cell and squamous cell carcinoma were evaluated independently by a dermatopathologist not otherwise involved with the for the presence of involucrin using immunoperoxidase study (Or. Theodore H. Kwan, Beth Israel Hospital, Boston, Massa techniques on paraffin sections. Basal cell carcinomas chusetts). Cases of basal cell carcinoma were subclassified according to were negative for involucrin with staining restricted to conve ntwnal histologic cri teria [10] into solid type (7 cases), superficial squanwus horn cysts, while squamous cell carcinomas multifocal (3 cases), adenoid (3 cases), sclerosing (4 cases), keratotic (:J stained strongly, particularly in large keratinized cells. -

432 Teams Dermatology
432 Teams Dermatology Structure and Function of the Skin Color Code: Original, Team’s note, Important, Doctor’s note, Not important, Old teamwork Done by: Mohammed Alshehri & Basil Al Suwaine Reviewer: Abdullah bin Saeed 4 Team Leader: Basil Al Suwaine 432 Dermatology Team Structure and Function of the Skin Objectives • To be familiar with the different structures of the skin. • To have basic knowledge of anatomy and function of the skin. • To be familiar with different tools to investigate skin disorders. • The relation between anatomy and diseases. • To have a general idea about different therapeutic options used in dermatology practice. P a g e | 1 432 Dermatology Team Structure and Function of the Skin Introduction The skin is the largest organ of the human body (1.75 m2), and the weight about 15% of the body It is divided into epidermis (ectoderm), dermis (mesoderm), subcutaneous fat and skin appendages (ectoderm and mesoderm). Dermal- Epidermal junction is called basement membrane, the weakest part in the skin and the usual site of blisters. Palms, soles, genitalia and scalp skin have slightly different structure. Useful video https://www.youtube.com/watch?v=z5VnOS9Ke3g P a g e | 2 432 Dermatology Team Structure and Function of the Skin The skin is a complex, dynamic organ. It is the largest organ of the body. It consists of many cell types and Specialized structures like “the Basement Membrane” It serves multiple functions that are crucial to health and survival. The skin consists of: Epidermis (has 4 layers) Basement membrane (between epidermis and dermis) thin 4 lyres Dermis (2 layers) Subcutaneous tissue Skin appendages Function: Prevent infections via innate and adaptive immunity (infections, autoimmunity, cancers). -

Table of Contents Previous Next Skin Is Composed of Two Layers, the Epidermis, the Outer Layer, Which Is Derived from Ectoderm
ATLAS OF HEAD AND NECK PATHOLOGY SKIN SKIN Skin is composed of two layers, the epidermis, the outer layer, which is derived from ectoderm, and the dermis, derived from mesoderm. The two layers are firmly adherent one to another with the dermis corresponding to the lamina propria of mucous membrane. Loose connective tissue lies under the dermis and corresponds to the superficial fascia. The epidermis is a stratified squamous keratinized epithelium with five layers or strata. (1) stratum germinativum or stratum basale lies just superficial to the dermis; (2) stratum spinosum or prickle cell layer; (3) stratum granulosum; (4) stratum lucidum; (5) stratum corneum. The stratum germinativum consists of a single layer of columnar or cuboidal cells. Mitotic figures are found here which produce new cells that eventually will be dis- placed into the next layer, the stratum spinosum, which is several layers thick and contains cells that are scale-like or polyhedral and which become flattened toward the surface. The name spinosum derives from “intercellular bridges” which appear to project from one cell to another but do not indicate cytoplasmic continuity between the cells The stratum spinosum and germinativum are grouped together as the malpighian layer and all cells here are referred to as keratinocytes. The stratum granulosum rests on top of the stratum spinosum and contains flattened polyhedral cells with vesicular nuclei. These cells contain granules of keratohyalin giving a granular appearance to the layer. The stratum lucidum is a clear translucent layer difficult to identify in many sections. The stratum corneum is the outermost layer of the epidermis and is composed of dead scale-like cells that become progressively flattened and joined together. -

Expression of Epithelial Antigens Exo-1 and EPM-1 in Human Epidermal Keratinocyte Maturation and Benign and Malignant Neoplasia1
[CANCER RESEARCH 50. 7668-7676. December I, 19901 Expression of Epithelial Antigens Exo-1 and EPM-1 in Human Epidermal Keratinocyte Maturation and Benign and Malignant Neoplasia1 Reinhard Klingel,2 Petra Boukamp, Roland Moll, Wolfgang Tilgen, Norbert E. Fusenig, Karl-Hermann Meyer zum Büschenfelde, and Wolfgang G. Dippold First Department of Internal Medicine, University of Mainz, D-6500 Main: [R. K., K-H. M. :. B., W. tÃ.I).I: Division of Differentiatinn and Carcinogenesis in Vitro, Institute of Biochemistry, Deutsches Krebsforschungs:entrum, D-6900 Heidelberg ¡P.B., N. E. F.J; Department of Pathology, University of Main:, D-6500 Mainz [R. M.I; and Department of Dermatology, University of Heidelberg, D-6900 Heidelberg ¡W.T.], Federal Republic ofdermany ABSTRACT lated but still tissue restricted (5-7). In human skin, proliferation and differentiation of keratino Exo-1, a polar neutral glycolipid, and EPM-1, a high molecular weight cytes and the development of benign and malignant neoplasia glycoprotein, are developmental antigens of human epithelial cells, ini are reflected by characteristic changes in tissue architecture. In tially described as components both on the cell surface and in secretions of gastrointestinal epithelia and respective tumors. In order to assess the turn, modifications in cell shape can act as a signal for terminal biological significance of both antigens for epithelial cell differentiation differentiation and inhibition of proliferation (8). Maturation and neoplastic transformation, their expression during human skin de and differentiation in human epidermis can be evaluated by velopment and benign and malignant neoplasia was analyzed in fresh determination of synthesis and modification of structural pro frozen tissue specimens of skin biopsies and of human epidermal keratin- teins and of alterations in the expression of cell surface antigens, ocytes growing in experimental model systems. -

Multiscale Modeling of Layer Formation in Epidermis Huijing Du University of Nebraska - Lincoln, [email protected]
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications, Department of Mathematics Mathematics, Department of 2018 Multiscale modeling of layer formation in epidermis Huijing Du University of Nebraska - Lincoln, [email protected] Yangyang Wang University of California, Irvine Daniel Haensel University of California, Irvine Briana Lee University of California, Irvine Xing Dai University of California, Irvine See next page for additional authors Follow this and additional works at: https://digitalcommons.unl.edu/mathfacpub Du, Huijing; Wang, Yangyang; Haensel, Daniel; Lee, Briana; Dai, Xing; and Nie, Qing, "Multiscale modeling of layer formation in epidermis" (2018). Faculty Publications, Department of Mathematics. 125. https://digitalcommons.unl.edu/mathfacpub/125 This Article is brought to you for free and open access by the Mathematics, Department of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Faculty Publications, Department of Mathematics by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Authors Huijing Du, Yangyang Wang, Daniel Haensel, Briana Lee, Xing Dai, and Qing Nie This article is available at DigitalCommons@University of Nebraska - Lincoln: https://digitalcommons.unl.edu/mathfacpub/125 RESEARCH ARTICLE Multiscale modeling of layer formation in epidermis Huijing Du1, Yangyang Wang2,3, Daniel Haensel3,4, Briana Lee3,4, Xing Dai3,4, Qing Nie2,3* 1 Department of Mathematics, University of Nebraska-Lincoln, Lincoln, -

A Human Keratin 14 ^'Knockout": the Absence of K14 Leads to Severe Epidermolysis Bullosa Simplex and a Function for an Intermediate Filament Protein
Downloaded from genesdev.cshlp.org on October 1, 2021 - Published by Cold Spring Harbor Laboratory Press A human keratin 14 ^'knockout": the absence of K14 leads to severe epidermolysis bullosa simplex and a function for an intermediate filament protein Yiu-mo Chan/ Ingrun Anton-Lamprecht/ Qian-Chun Yu/ Andreas Jackel/ Bernhard Zabel/ Jan-Peter Ernst/ and Elaine Fuchs^'^ ^Howard Hughes Medical Institute, Department of Molecular Genetics and Cell Biology, The University of Chicago, Chicago, Illinois 60637 USA; ^Institut fur Ultrastrukturforschung der Haut, Universitats-Hautklinik, D-69115 Heidelberg, Germany; ^Department of Pediatrics, University of Mainz, D-55131 Mainz, Germany; "^Department of Pediatrics, Klinikum Wiesbaden, D-65119, Wiesbaden, Germany Since their discovery, the hinction of intermediate filaments (IFs) has remained obscure. In skin, epidermal cells have extensive cytoskeletal architectures of IFs, composed of type I and type II keratin heterodimers. Clues to possible functions of these proteins have come from recent studies showing that several autosomal-dominant, blistering skin disorders are caused by defects in genes that encode epidermal keratins. These diseases all exhibit cell degeneration and keratin network perturbations in cells that express the particular mutant keratin gene. However, it is not clear from these studies whether cytolysis arises from the presence of large insoluble keratin aggregates that compromise cellular physiology or from the absence of an extensive keratin filament network, which jeopardizes mechanical integrity. We report here the analysis of an extremely rare case of severe recessive epidermolysis bullosa simplex (EBS), where the patient lacks a discernible keratin filament network in basal epidermal cells. Genetic analyses revealed a homozygous point mutation that yielded a premature termination codon in the major basal type I keratin gene and caused complete ablation of K14. -
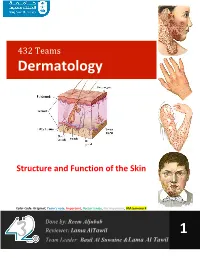
Epidermis (Ectoderm), Dermis (Mesoderm), Subcutaneous Fat and Skin Appendages (Ectoderm and Mesoderm)
432 Teams Dermatology Structure and Function of the Skin Color Code: Original, Team’s note, Important, Doctor’s note, Not important, Old teamwork Done by: Reem Aljubab Reviewer: Lama AlTawil 1 Team Leader: Basil Al Suwaine &Lama Al Tawil 432 Dermatology Team Structure and Function of the Skin Objectives • To be familiar with the different structures of the skin. • To have basic knowledge of anatomy and function of the skin. • To be familiar with different tools to investigate skin disorders. • The relation between anatomy and diseases. • To have a general idea about different therapeutic options used in dermatology practice. Lecture outline Function , Structure of the skin. Approach to dermatology patient. Descriptive terms and morphology of skin lesions. Reaction patterns. Topical therapy and others. P a g e | 1 432 Dermatology Team Structure and Function of the Skin Introduction to Dermatology -The skin is a complex, dynamic organ. -It is the largest organ of the body and weighs about 15% of the body -It consist of many cell types called Keratinocytes ( building block) - Specialized structures like the Basement Membrane. -It serves multiple functions that are crucial to health and survival. -It is divided into epidermis (ectoderm), dermis (mesoderm), subcutaneous fat and skin appendages (ectoderm and mesoderm). -Dermal-Epidermal junction is called basement membrane, the weakest part in the skin and the usual site of blisters. Video:https://www.youtube.com/watch?v=z5VnOS9Ke3g P a g e | 2 432 Dermatology Team Structure and Function -

Multiscale Modeling of Layer Formation in Epidermis
RESEARCH ARTICLE Multiscale modeling of layer formation in epidermis Huijing Du1, Yangyang Wang2,3, Daniel Haensel3,4, Briana Lee3,4, Xing Dai3,4, Qing Nie2,3* 1 Department of Mathematics, University of Nebraska-Lincoln, Lincoln, NE, United States of America, 2 Department of Mathematics, University of California Irvine, Irvine, CA, United States of America, 3 Center for Complex Biological Systems, University of California Irvine, Irvine, CA, United States of America, 4 Department of Biological Chemistry, School of Medicine, University of California Irvine, Irvine, CA, United States of America a1111111111 * [email protected] a1111111111 a1111111111 a1111111111 Abstract a1111111111 The mammalian skin epidermis is a stratified epithelium composed of multiple layers of epi- thelial cells that exist in appropriate sizes and proportions, and with distinct boundaries sep- arating each other. How the epidermis develops from a single layer of committed precursor OPEN ACCESS cells to form a complex multilayered structure of multiple cell types remains elusive. Here, Citation: Du H, Wang Y, Haensel D, Lee B, Dai X, we construct stochastic, three-dimensional, and multiscale models consisting of a lineage of Nie Q (2018) Multiscale modeling of layer multiple cell types to study the control of epidermal development. Symmetric and asymmet- formation in epidermis. PLoS Comput Biol 14(2): ric cell divisions, stochastic cell fate transitions within the lineage, extracellular morphogens, e1006006. https://doi.org/10.1371/journal. cell-to-cell adhesion forces, and cell signaling are included in model. A GPU algorithm was pcbi.1006006 developed and implemented to accelerate the simulations. These simulations show that a Editor: Philip K. -

United States Patent (19) 11 Patent Number: 5,912,175 Wille, Jr
USOO591.2175A United States Patent (19) 11 Patent Number: 5,912,175 Wille, Jr. (45) Date of Patent: Jun. 15, 1999 54 PROCESS AND MEDIA FOR THE GROWTH 52 U.S. Cl. .......................... 435/405; 435/325; 435/383; OF HUMAN CORNEA AND GNGIWA 435/384; 435/404 58 Field of Search ..................................... 435/405, 325, 75 Inventor: John J. Wille, Jr., Trenton, N.J. 435/383,384, 404 73 Assignee: Hy-Gene, Inc., Ventura, Calif. Primary Examiner Leon B. Lankford, Jr. 21 Appl. No.: 09/133,386 Attorney, Agent, or Firm Donald O. Nickey; Standley & Gilcrest 22 Filed: Aug. 13, 1998 57 ABSTRACT Related U.S. Application Data Media and methods are disclosed for the in vitro formation 63 Continuation of application No. 08/893,195, Jul. 15, 1997, of a histologically complete human epithelium. The media Pat. No. 5,834.312, which is a continuation-in-part of appli are Serum-free, companion cell or feeder layer free and cation No. 08/500,744, Jul. 11, 1995, Pat. No. 5,686,307, organotypic, matrix free Solutions for the isolation and which is a continuation of application No. 08/318,221, Oct. cultivation of clonally competent basal epithelial cells. The 5, 1994, abandoned, which is a continuation of application No. 08/184,905, Jan. 21, 1994, abandoned, which is a media and methods of the invention are useful in the continuation of application No. 08/063,247, May 18, 1993, production of epithelial tissueS Such as epidermis, cornea, abandoned, which is a division of application No. 07/471, gingiva and ureter.