Reconstructing Dipsacales Phylogeny Using Angiosperms353: Issues and Insights
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Toward a Resolution of Campanulid Phylogeny, with Special Reference to the Placement of Dipsacales
TAXON 57 (1) • February 2008: 53–65 Winkworth & al. • Campanulid phylogeny MOLECULAR PHYLOGENETICS Toward a resolution of Campanulid phylogeny, with special reference to the placement of Dipsacales Richard C. Winkworth1,2, Johannes Lundberg3 & Michael J. Donoghue4 1 Departamento de Botânica, Instituto de Biociências, Universidade de São Paulo, Caixa Postal 11461–CEP 05422-970, São Paulo, SP, Brazil. [email protected] (author for correspondence) 2 Current address: School of Biology, Chemistry, and Environmental Sciences, University of the South Pacific, Private Bag, Laucala Campus, Suva, Fiji 3 Department of Phanerogamic Botany, The Swedish Museum of Natural History, Box 50007, 104 05 Stockholm, Sweden 4 Department of Ecology & Evolutionary Biology and Peabody Museum of Natural History, Yale University, P.O. Box 208106, New Haven, Connecticut 06520-8106, U.S.A. Broad-scale phylogenetic analyses of the angiosperms and of the Asteridae have failed to confidently resolve relationships among the major lineages of the campanulid Asteridae (i.e., the euasterid II of APG II, 2003). To address this problem we assembled presently available sequences for a core set of 50 taxa, representing the diver- sity of the four largest lineages (Apiales, Aquifoliales, Asterales, Dipsacales) as well as the smaller “unplaced” groups (e.g., Bruniaceae, Paracryphiaceae, Columelliaceae). We constructed four data matrices for phylogenetic analysis: a chloroplast coding matrix (atpB, matK, ndhF, rbcL), a chloroplast non-coding matrix (rps16 intron, trnT-F region, trnV-atpE IGS), a combined chloroplast dataset (all seven chloroplast regions), and a combined genome matrix (seven chloroplast regions plus 18S and 26S rDNA). Bayesian analyses of these datasets using mixed substitution models produced often well-resolved and supported trees. -

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

Heptacodium Miconioides
photograph © Peter Del Tredici, Arnold Arboretum of Harvard University An old multi-stemmed specimen of Heptacodium miconioides growing in woodland on Tian Tai Shan, Zhejiang, September 2004, probably the first wild plant ofHeptacodium to be seen by a Western botanist since its discovery by Ernest Wilson in 1907. Heptacodium miconioides Rehder In his last ‘Tree of the Year’, JOHN GRIMSHAW discusses what distinguishes a shrub from a tree and writes about a large shrub introduced to cultivation first in the early twentieth century and again in the late twentieth century, that is rare in the wild. Introduction When the terms of reference for New Trees were drawn up (see pp. 1-2), having agreed that the book would contain only trees, we had to address the deceptive question ‘what is a tree?’ Most of us ‘can recognise a tree when we see one’ and in many ways this is as good an answer as one needs, but when is a woody plant not a tree? When it’s a shrub, of course – but what is a shrub? In the end we adopted the simple definitions that a tree ‘normally [has] a single stem reaching or exceeding 5 m in height, at least in its native habitat. A shrub would normally have multiple woody stems emerging from the base or close to the ground, and would seldom exceed 5 m in height’ (Grimshaw & Bayton 2009). In most cases this served to make a clear distinction, but one of the casualties that fell between the two stools was the Chinese plant Heptacodium miconioides, a significant recent introduction described by theFlora of China (Yang et al. -

Plastid Phylogenomic Insights Into the Evolution of the Caprifoliaceae S.L. (Dipsacales)
Molecular Phylogenetics and Evolution 142 (2020) 106641 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Plastid phylogenomic insights into the evolution of the Caprifoliaceae s.l. T (Dipsacales) Hong-Xin Wanga,1, Huan Liub,c,1, Michael J. Moored, Sven Landreine, Bing Liuf,g, Zhi-Xin Zhua, ⁎ Hua-Feng Wanga, a Key Laboratory of Tropical Biological Resources of Ministry of Education, School of Life and Pharmaceutical Sciences, Hainan University, Haikou 570228, China b BGI-Shenzhen, Beishan Industrial Zone, Yantian District, Shenzhen 518083, China c State Key Laboratory of Agricultural Genomics, BGI-Shenzhen, Shenzhen 518083, China d Department of Biology, Oberlin College, Oberlin, OH 44074, USA e Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Menglun, 666303, China f State Key Laboratory of Systematic and Evolutionary Botany, Institute of Botany, Chinese Academy of Science, Beijing 100093, China g Sino-African Joint Research Centre, Chinese Academy of Science, Wuhan 430074, China ARTICLE INFO ABSTRACT Keywords: The family Caprifoliaceae s.l. is an asterid angiosperm clade of ca. 960 species, most of which are distributed in Caprifoliaceae s.l. temperate regions of the northern hemisphere. Recent studies show that the family comprises seven major Dipsacales clades: Linnaeoideae, Zabelia, Morinoideae, Dipsacoideae, Valerianoideae, Caprifolioideae, and Diervilloideae. Plastome However, its phylogeny at the subfamily or genus level remains controversial, and the backbone relationships Phylogenetics among subfamilies are incompletely resolved. In this study, we utilized complete plastome sequencing to resolve the relationships among the subfamilies of the Caprifoliaceae s.l. and clarify several long-standing controversies. We generated and analyzed plastomes of 48 accessions of Caprifoliaceae s.l., representing 44 species, six sub- families and one genus. -

The Role of Species Including in Valerianella Genus in Phytocenoses in the Area of Nakhchivan Autonomous Republic // Бюллетень Науки И Практики
Бюллетень науки и практики / Bulletin of Science and Practice Т. 6. №7. 2020 https://www.bulletennauki.com https://doi.org/10.33619/2414-2948/56 UDC 635.57 https://doi.org/10.33619/2414-2948/56/04 AGRIS F40 THE ROLE OF SPECIES INCLUDING IN VALERIANELLA L. GENUS IN PHYTOCENOSES IN THE AREA OF NAKHCHIVAN AUTONOMOUS REPUBLIC ©Talibov T., Academician of Azerbaijan NAS, Dr. habil., Institute of Bioresources Nakhchivan branch of Azerbaijan NAS, Nakhchivan, Azerbaijan, [email protected] ©Mahmudova U., ORCID: 0000-0003-2877-3761, Nakhchivan State University, Nakhchivan, Azerbaijan, [email protected] РОЛЬ ВИДОВ РОДА VALERIANELLA L., ВХОДЯЩИХ В НЕКОТОРЫЕ ФИТОЦЕНОЗЫ НАХИЧЕВАНСКОЙ АВТОНОМНОЙ РЕСПУБЛИКИ ©Талыбов Т. Г., акад. НАН Азербайджана, д-р биол. наук, Институт биоресурсов Нахичеванского отделения НАН Азербайджана, г. Нахичевань, Азербайджан, [email protected] ©Махмудова У. М., ORCID: 0000-0003-2877-3761, Нахичеванский государственный университет, г. Нахичевань, Азербайджан, [email protected] Abstract. In this article the structure of species including in Valerianella Genus was studied with geobotanical methods, their role in phytocenosis, their formations, and associations were determined in the vegetation cover in Soyugdagh and Khorhat mountainous areas of Ordubad region. It has been determined that although the species of the Valerianella Genus are small numbers in phytocenosis and associations, they have great importance in improving the fodder quality of the vegetation. This plays an important role in the enrichment of food ration of Bezoar Goat and other herbivorous animals that inhabit the area of Zangezur National Park. Аннотация. В данной статье изучена структура видов рода Valerianella геоботаническими методами, определена их роль в фитоценозе, определены их формации и ассоциации в растительном покрове горных районов Союгдаг и Хорхат Ордубадского района. -

Towards an Updated Checklist of the Libyan Flora
Towards an updated checklist of the Libyan flora Article Published Version Creative Commons: Attribution 3.0 (CC-BY) Open access Gawhari, A. M. H., Jury, S. L. and Culham, A. (2018) Towards an updated checklist of the Libyan flora. Phytotaxa, 338 (1). pp. 1-16. ISSN 1179-3155 doi: https://doi.org/10.11646/phytotaxa.338.1.1 Available at http://centaur.reading.ac.uk/76559/ It is advisable to refer to the publisher’s version if you intend to cite from the work. See Guidance on citing . Published version at: http://dx.doi.org/10.11646/phytotaxa.338.1.1 Identification Number/DOI: https://doi.org/10.11646/phytotaxa.338.1.1 <https://doi.org/10.11646/phytotaxa.338.1.1> Publisher: Magnolia Press All outputs in CentAUR are protected by Intellectual Property Rights law, including copyright law. Copyright and IPR is retained by the creators or other copyright holders. Terms and conditions for use of this material are defined in the End User Agreement . www.reading.ac.uk/centaur CentAUR Central Archive at the University of Reading Reading’s research outputs online Phytotaxa 338 (1): 001–016 ISSN 1179-3155 (print edition) http://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2018 Magnolia Press Article ISSN 1179-3163 (online edition) https://doi.org/10.11646/phytotaxa.338.1.1 Towards an updated checklist of the Libyan flora AHMED M. H. GAWHARI1, 2, STEPHEN L. JURY 2 & ALASTAIR CULHAM 2 1 Botany Department, Cyrenaica Herbarium, Faculty of Sciences, University of Benghazi, Benghazi, Libya E-mail: [email protected] 2 University of Reading Herbarium, The Harborne Building, School of Biological Sciences, University of Reading, Whiteknights, Read- ing, RG6 6AS, U.K. -

Botanic Gardens and Their Contribution to Sustainable Development Goal 15 - Life on Land Volume 15 • Number 2
Journal of Botanic Gardens Conservation International Volume 15 • Number 2 • July 2018 Botanic gardens and their contribution to Sustainable Development Goal 15 - Life on Land Volume 15 • Number 2 IN THIS ISSUE... EDITORS EDITORIAL: BOTANIC GARDENS AND SUSTAINABLE DEVELOPMENT GOAL 15 .... 02 FEATURES NEWS FROM BGCI .... 04 Suzanne Sharrock Paul Smith Director of Global Secretary General Programmes PLANT HUNTING TALES: SEED COLLECTING IN THE WESTERN CAPE OF SOUTH AFRICA .... 06 Cover Photo: Franklinia alatamaha is extinct in the wild but successfully grown in botanic gardens and arboreta FEATURED GARDEN: SOUTH AFRICA’S NATIONAL BOTANICAL GARDENS .... 09 (Arboretum Wespelaar) Design: Seascape www.seascapedesign.co.uk INTERVIEW: TALKING PLANTS .... 12 BGjournal is published by Botanic Gardens Conservation International (BGCI). It is published twice a year. Membership is open to all interested individuals, institutions and organisations that support the aims of BGCI. Further details available from: • Botanic Gardens Conservation International, Descanso ARTICLES House, 199 Kew Road, Richmond, Surrey TW9 3BW UK. Tel: +44 (0)20 8332 5953, Fax: +44 (0)20 8332 5956, E-mail: [email protected], www.bgci.org SUSTAINABLE DEVELOPMENT GOAL 15 • BGCI (US) Inc, The Huntington Library, Suzanne Sharrock .... 14 Art Collections and Botanical Gardens, 1151 Oxford Rd, San Marino, CA 91108, USA. Tel: +1 626-405-2100, E-mail: [email protected] SDG15: TARGET 15.1 Internet: www.bgci.org/usa AUROVILLE BOTANICAL GARDENS – CONSERVING TROPICAL DRY • BGCI (China), South China Botanical Garden, EVERGREEN FOREST IN INDIA 1190 Tian Yuan Road, Guangzhou, 510520, China. Paul Blanchflower .... 16 Tel: +86 20 85231992, Email: [email protected], Internet: www.bgci.org/china SDG 15: TARGET 15.3 • BGCI (Southeast Asia), Jean Linsky, BGCI Southeast Asia REVERSING LAND DEGRADATION AND DESERTIFICATION IN Botanic Gardens Network Coordinator, Dr. -

Phylogeny and Phylogenetic Taxonomy of Dipsacales, with Special Reference to Sinadoxa and Tetradoxa (Adoxaceae)
PHYLOGENY AND PHYLOGENETIC TAXONOMY OF DIPSACALES, WITH SPECIAL REFERENCE TO SINADOXA AND TETRADOXA (ADOXACEAE) MICHAEL J. DONOGHUE,1 TORSTEN ERIKSSON,2 PATRICK A. REEVES,3 AND RICHARD G. OLMSTEAD 3 Abstract. To further clarify phylogenetic relationships within Dipsacales,we analyzed new and previously pub- lished rbcL sequences, alone and in combination with morphological data. We also examined relationships within Adoxaceae using rbcL and nuclear ribosomal internal transcribed spacer (ITS) sequences. We conclude from these analyses that Dipsacales comprise two major lineages:Adoxaceae and Caprifoliaceae (sensu Judd et al.,1994), which both contain elements of traditional Caprifoliaceae.Within Adoxaceae, the following relation- ships are strongly supported: (Viburnum (Sambucus (Sinadoxa (Tetradoxa, Adoxa)))). Combined analyses of C ap ri foliaceae yield the fo l l ow i n g : ( C ap ri folieae (Diervilleae (Linnaeeae (Morinaceae (Dipsacaceae (Triplostegia,Valerianaceae)))))). On the basis of these results we provide phylogenetic definitions for the names of several major clades. Within Adoxaceae, Adoxina refers to the clade including Sinadoxa, Tetradoxa, and Adoxa.This lineage is marked by herbaceous habit, reduction in the number of perianth parts,nectaries of mul- ticellular hairs on the perianth,and bifid stamens. The clade including Morinaceae,Valerianaceae, Triplostegia, and Dipsacaceae is here named Valerina. Probable synapomorphies include herbaceousness,presence of an epi- calyx (lost or modified in Valerianaceae), reduced endosperm,and distinctive chemistry, including production of monoterpenoids. The clade containing Valerina plus Linnaeeae we name Linnina. This lineage is distinguished by reduction to four (or fewer) stamens, by abortion of two of the three carpels,and possibly by supernumerary inflorescences bracts. Keywords: Adoxaceae, Caprifoliaceae, Dipsacales, ITS, morphological characters, phylogeny, phylogenetic taxonomy, phylogenetic nomenclature, rbcL, Sinadoxa, Tetradoxa. -
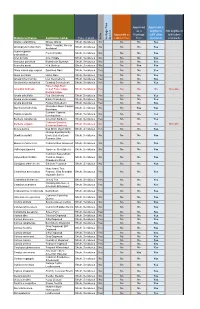
Botanical Name Common Name
Approved Approved & as a eligible to Not eligible to Approved as Frontage fulfill other fulfill other Type of plant a Street Tree Tree standards standards Heritage Tree Tree Heritage Species Botanical Name Common name Native Abelia x grandiflora Glossy Abelia Shrub, Deciduous No No No Yes White Forsytha; Korean Abeliophyllum distichum Shrub, Deciduous No No No Yes Abelialeaf Acanthropanax Fiveleaf Aralia Shrub, Deciduous No No No Yes sieboldianus Acer ginnala Amur Maple Shrub, Deciduous No No No Yes Aesculus parviflora Bottlebrush Buckeye Shrub, Deciduous No No No Yes Aesculus pavia Red Buckeye Shrub, Deciduous No No Yes Yes Alnus incana ssp. rugosa Speckled Alder Shrub, Deciduous Yes No No Yes Alnus serrulata Hazel Alder Shrub, Deciduous Yes No No Yes Amelanchier humilis Low Serviceberry Shrub, Deciduous Yes No No Yes Amelanchier stolonifera Running Serviceberry Shrub, Deciduous Yes No No Yes False Indigo Bush; Amorpha fruticosa Desert False Indigo; Shrub, Deciduous Yes No No No Not eligible Bastard Indigo Aronia arbutifolia Red Chokeberry Shrub, Deciduous Yes No No Yes Aronia melanocarpa Black Chokeberry Shrub, Deciduous Yes No No Yes Aronia prunifolia Purple Chokeberry Shrub, Deciduous Yes No No Yes Groundsel-Bush; Eastern Baccharis halimifolia Shrub, Deciduous No No Yes Yes Baccharis Summer Cypress; Bassia scoparia Shrub, Deciduous No No No Yes Burning-Bush Berberis canadensis American Barberry Shrub, Deciduous Yes No No Yes Common Barberry; Berberis vulgaris Shrub, Deciduous No No No No Not eligible European Barberry Betula pumila -

Number 3, Spring 1998 Director’S Letter
Planning and planting for a better world Friends of the JC Raulston Arboretum Newsletter Number 3, Spring 1998 Director’s Letter Spring greetings from the JC Raulston Arboretum! This garden- ing season is in full swing, and the Arboretum is the place to be. Emergence is the word! Flowers and foliage are emerging every- where. We had a magnificent late winter and early spring. The Cornus mas ‘Spring Glow’ located in the paradise garden was exquisite this year. The bright yellow flowers are bright and persistent, and the Students from a Wake Tech Community College Photography Class find exfoliating bark and attractive habit plenty to photograph on a February day in the Arboretum. make it a winner. It’s no wonder that JC was so excited about this done soon. Make sure you check of themselves than is expected to seedling selection from the field out many of the special gardens in keep things moving forward. I, for nursery. We are looking to propa- the Arboretum. Our volunteer one, am thankful for each and every gate numerous plants this spring in curators are busy planting and one of them. hopes of getting it into the trade. preparing those gardens for The magnolias were looking another season. Many thanks to all Lastly, when you visit the garden I fantastic until we had three days in our volunteers who work so very would challenge you to find the a row of temperatures in the low hard in the garden. It shows! Euscaphis japonicus. We had a twenties. There was plenty of Another reminder — from April to beautiful seven-foot specimen tree damage to open flowers, but the October, on Sunday’s at 2:00 p.m. -

Joint City Council / Planning Commission Work Session Meeting
City of North Plains Agenda Joint City Council / Planning Commission Work Session Meeting Thursday, September 12, 2019 @ 5:30 PM North Plains Senior Center 31450 NW Commercial Street Page 1. CALL TO ORDER 2. PLEDGE OF ALLEGIANCE 3. ROLL CALL 4. NEW BUSINESS: A. Discussion on Community Pattern Books for the Brynhill Subdivision 2 - 44 Rudy Kadlub - Pacific Costa □ Brynhill Community Elements Book 2019-08-29 □ Brynhill Community Pattern Book 2019-08-29 5. ADJOURNMENT: ***** North Plains City Council meetings are accessible for disabled individuals. The City will also endeavor to provide services for persons with impaired hearing or vision and other services, if requested, at least 48 hours prior to the meeting. To obtain services, please call City Hall at (503) 647-5555 ***** NORTH PLAINS JOINT CITY COUNCIL / PLANNING COMMISSION WORK SESSION AGENDA PACKET Thursday, September 12, 2019 Page 1 of 44 Page 2 of 44 Community Elements Book Brynhill Page 3 of 44 This Community Elements Book is a key component in the Brynhill community. This plan identifies plants, street trees, lighting, play structures and site furnishings to be used throughout all phases within the Brynhill Community. All submissions and revisions to the plants, street trees, lighting, play structures, and site furnishings will be reviewed for compliance by the Master Planner Rudy Kadlub of Costa Pacific Communities with Lee Iverson of Iverson Architects as successor, or as assigned by City and approved by Rudy Kadlub or Lee Iverson. TABLE OF CONTENTS Table of Contents 2 Fencing -

(Lonicera L.) Genties Atstovų Genetinės Įvairovės Ir Filogenetiniai Tyrimai Dnr Ţymenų Metodais
VILNIAUS UNIVERSITETAS Donatas Naugţemys SAUSMEDŢIO (LONICERA L.) GENTIES ATSTOVŲ GENETINĖS ĮVAIROVĖS IR FILOGENETINIAI TYRIMAI DNR ŢYMENŲ METODAIS Daktaro disertacija Biomedicinos mokslai, biologija (01 B) Vilnius, 2011 Disertacija rengta 2006 – 2010 metais Vilniaus universitete. Mokslinis vadovas: prof. dr. Donatas Ţvingila (Vilniaus universitetas, biomedicinos mokslai, biologija – 01 B) Konsultantas: dr. Silva Ţilinskaitė (Vilniaus universitetas, biomedicinos mokslai, biologija – 01 B) 2 TURINYS SANTRUMPOS ..................................................................................................... 5 ĮVADAS ................................................................................................................. 7 I. LITERATŪROS APŢVALGA ......................................................................... 13 1. Sausmedţio genties apţvalga ....................................................................... 13 1.1. Lonicera L. genties sistematikos istorija ir problemos .......................... 15 1.2. Lonicera L. genties kilmė ...................................................................... 21 2. Molekuliniai ţymenys ir augalų filogenetiniai tyrimai ................................ 24 2.1. RAPD metodo taikymas augalų sistematikoje ...................................... 26 2.2. Chloroplastų DNR nekoduojančių specifinių regionų tyrimas sekoskaitos metodu .............................................................................................. 31 2.3. Lonicera L. genties filogenetikos molekuliniai tyrimai