Neural Processing of Musical Features A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Detecting Harmonic Change in Musical Audio
Detecting Harmonic Change In Musical Audio Christopher Harte and Mark Sandler Martin Gasser Centre for Digital Music Austrian Research Institute for Artificial Queen Mary, University of London Intelligence(OF¨ AI) London, UK Freyung 6/6, 1010 Vienna, Austria [email protected] [email protected] ABSTRACT We propose a novel method for detecting changes in the harmonic content of musical audio signals. Our method uses a new model for Equal Tempered Pitch Class Space. This model maps 12-bin chroma vectors to the interior space of a 6-D polytope; pitch classes are mapped onto the vertices of this polytope. Close harmonic relations such as fifths and thirds appear as small Euclidian distances. We calculate the Euclidian distance between analysis frames n + 1 and n − 1 to develop a harmonic change measure for frame n. A peak in the detection function denotes a transi- tion from one harmonically stable region to another. Initial experiments show that the algorithm can successfully detect harmonic changes such as chord boundaries in polyphonic audio recordings. Categories and Subject Descriptors J.0 [Computer Applications]: General General Terms Figure 1: Flow Diagram of HCDF System. Algorithms, Theory has many potential applications in the segmentation of au- dio signals, particularly as a preprocessing stage for further Keywords harmonic recognition and classification algorithms. The pri- Pitch Space, Harmonic, Segmentation, Music, Audio mary motivation for this work is for use in chord recogni- tion from audio. Used as a segmentation algorithm, this approach provides a good foundation for solving the general 1. INTRODUCTION chord recognition problem of needing to know the positions In this paper we introduce a novel process for detect- of chord boundaries in the audio data before being able to ing changes in the harmonic content of audio signals. -

Melodies in Space: Neural Processing of Musical Features A
Melodies in space: Neural processing of musical features A DISSERTATION SUBMITTED TO THE FACULTY OF THE GRADUATE SCHOOL OF THE UNIVERSITY OF MINNESOTA BY Roger Edward Dumas IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY Dr. Apostolos P. Georgopoulos, Adviser Dr. Scott D. Lipscomb, Co-adviser March 2013 © Roger Edward Dumas 2013 Table of Contents Table of Contents .......................................................................................................... i Abbreviations ........................................................................................................... xiv List of Tables ................................................................................................................. v List of Figures ............................................................................................................. vi List of Equations ...................................................................................................... xiii Chapter 1. Introduction ............................................................................................ 1 Melody & neuro-imaging .................................................................................................. 1 The MEG signal ................................................................................................................................. 3 Background ........................................................................................................................... 6 Melodic pitch -
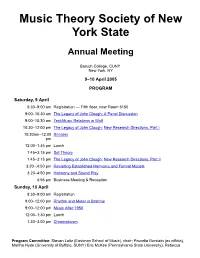
Program and Abstracts for 2005 Meeting
Music Theory Society of New York State Annual Meeting Baruch College, CUNY New York, NY 9–10 April 2005 PROGRAM Saturday, 9 April 8:30–9:00 am Registration — Fifth floor, near Room 5150 9:00–10:30 am The Legacy of John Clough: A Panel Discussion 9:00–10:30 am TextMusic Relations in Wolf 10:30–12:00 pm The Legacy of John Clough: New Research Directions, Part I 10:30am–12:00 Scriabin pm 12:00–1:45 pm Lunch 1:45–3:15 pm Set Theory 1:45–3:15 pm The Legacy of John Clough: New Research Directions, Part II 3:20–;4:50 pm Revisiting Established Harmonic and Formal Models 3:20–4:50 pm Harmony and Sound Play 4:55 pm Business Meeting & Reception Sunday, 10 April 8:30–9:00 am Registration 9:00–12:00 pm Rhythm and Meter in Brahms 9:00–12:00 pm Music After 1950 12:00–1:30 pm Lunch 1:30–3:00 pm Chromaticism Program Committee: Steven Laitz (Eastman School of Music), chair; Poundie Burstein (ex officio), Martha Hyde (University of Buffalo, SUNY) Eric McKee (Pennsylvania State University); Rebecca Jemian (Ithaca College), and Alexandra Vojcic (Juilliard). MTSNYS Home Page | Conference Information Saturday, 9:00–10:30 am The Legacy of John Clough: A Panel Discussion Chair: Norman Carey (Eastman School of Music) Jack Douthett (University at Buffalo, SUNY) Nora Engebretsen (Bowling Green State University) Jonathan Kochavi (Swarthmore, PA) Norman Carey (Eastman School of Music) John Clough was a pioneer in the field of scale theory. -

Music and Operations Research - the Perfect Match?
This article grew out of an invited lecture at the University of Massachusetts, Amherst, Operations Research / Management Science Seminar Series organized by Anna Nagurney, 2005-2006 fellow at the Radcliffe Institute for Advanced Study, and the UMass - Amherst INFORMS Student Chapter. Music and Operations Research - The Perfect Match? By Elaine Chew Operations research (OR) is a field that prides itself in its be a musical Google, whereby one could retrieve music files by versatility in the mathematical modeling of complex problems to humming a melody or by providing an audio sample. find optimal or feasible solutions. It should come as no surprise Beyond such industrial interests in mathematical and that OR has much to offer in terms of solving problems in music computational modeling of music, such approaches to solving problems composition, analysis, and performance. Operations researchers in music analysis and in music making have their own scientific and have tackled problems in areas ranging from airline yield intellectual merit as a natural progression in the evolution of the management and computational finance, to computational disciplines of musicology, music theory, performance, composition, and biology and radiation oncology, to computational linguistics, and improvisation. Formal models of human capabilities in creating, in eclectic applications such as diet formulation, sports strategies analyzing, and reproducing music serve to further knowledge in human and chicken dominance behavior, so why not music? perception and cognition, and advance the state of the art in psychology I am an operations researcher and a musician. Over the past ten and neuroscience. By modeling music making and analysis, we gain a years, I have devoted much time to exploring, and building a career at, deeper understanding of the levels and kinds of human creativity the interface between music and operations research. -

Messiaen's Regard IV: Automatic Segmentation Using the Spiral Array
REGARDS ON TWO REGARDS BY MESSIAEN: AUTOMATIC SEGMENTATION USING THE SPIRAL ARRAY Elaine Chew University of Southern California Viterbi School of Engineering Epstein Department of Industrial and Systems Engineering Integrated Media Systems Center [email protected] ABSTRACT the same space and uses spatial points in the model’s interior to summarize and represent segments of music. Segmentation by pitch context is a fundamental process The array of pitch representations in the Spiral Array is in music cognition, and applies to both tonal and atonal akin to Longuet-Higgins’ Harmonic Network [7] and the music. This paper introduces a real-time, O(n), tonnetz of neo-Riemannian music theory [4]. The key algorithm for segmenting music automatically by pitch spirals, generated by mathematical aggregation, collection using the Spiral Array model. The correspond in structure to Krumhansl’s network of key segmentation algorithm is applied to Olivier Messiaen’s relations [5] and key representations in Lerdahl’s tonal Regard de la Vierge (Regard IV) and Regard des pitch space [6], each modeled using entirely different prophètes, des bergers et des Mages (Regard XVI) from approaches. his Vingt Regards sur l’Enfant Jésus. The algorithm The present segmentation algorithm, named Argus, is uses backward and forward windows at each point in the latest in a series of computational analysis techniques time to capture local pitch context in the recent past and utilizing the Spiral Array. The Spiral Array model has not-too-distant future. The content of each window is been used in the design of algorithms for key-finding [2] mapped to a spatial point, called the center of effect and off-line determining of key boundaries [3], among (c.e.), in the interior of the Spiral Array. -

A Multi-Level Tonal Interval Space for Modelling Pitch Relatedness and Musical Consonance
Journal of New Music Research, 2016 Vol. 45, No. 4, 281–294, http://dx.doi.org/10.1080/09298215.2016.1182192 A multi-level tonal interval space for modelling pitch relatedness and musical consonance Gilberto Bernardes1 ,DiogoCocharro1,MarceloCaetano1 ,CarlosGuedes1,2 and Matthew E.P. Davies1 1INESC TEC, Portugal; 2New York University Abu Dhabi, United Arab Emirates (Received 12 March 2015; accepted 18 April 2016) Abstract The intelligibility and high explanatory power of tonal pitch spaces usually hide complex theories, which need to account In this paper we present a 12-dimensional tonal space in the for a variety of subjective and contextual factors. To a certain context of the ,Chew’sSpiralArray,andHarte’s Tonnetz extent, the large number of different, and sometimes con- 6-dimensional Tonal Centroid Space. The proposed Tonal tradictory, tonal pitch spaces presented in the literature help Interval Space is calculated as the weighted Discrete Fourier us understand the complexity of such representations. Exist- Transform of normalized 12-element chroma vectors, which ing tonal spaces can be roughly divided into two categories, we represent as six circles covering the set of all possible pitch each anchored to a specific discipline and applied methods. intervals in the chroma space. By weighting the contribution On the one hand we have models grounded in music theory of each circle (and hence pitch interval) independently, we (Cohn, 1997, 1998;Lewin,1987;Tymoczko,2011; Weber, can create a space in which angular and Euclidean distances 1817–1821), and on the other hand, models based on cogni- among pitches, chords, and regions concur with music theory tive psychology (Krumhansl, 1990;Longuet-Higgins,1962; principles. -

Visualization of Low Dimensional Structure in Tonal Pitch Space
VISUALIZATION OF LOW DIMENSIONAL STRUCTURE IN TONAL PITCH SPACE J. Ashley Burgoyne and Lawrence K. Saul Department of Computer and Information Science University of Pennsylvania {burgoyne,lsaul}@cis.upenn.edu ABSTRACT d] F] f] A a C c g] B b D d F f In his 2001 monograph Tonal Pitch Space, Fred Ler- dahl defined an distance function over tonal and post-tonal c] E e G g B[ b[ harmonies distilled from years of research on music cog- nition. Although this work references the toroidal struc- f] A a C c E[ e[ ture commonly associated with harmonic space, it stops b D d F f A[ a[ short of presenting an explicit embedding of this torus. It is possible to use statistical techniques to recreate such an e G g B[ b[ D[ d[ embedding from the distance function, yielding a more a C c E[ e[ G[ g[ complex structure than the standard toroidal model has heretofore assumed. Nonlinear techniques can reduce the Figure 1. Weber’s diagram of tonal space dimensionality of this structure and be tuned to emphasize global or local structure. The resulting manifolds high- light the relationships inherent in the tonal system and of- over harmonies. No other research to date has attempted fer a basis for future work in machine-assisted analysis to embed it explicitly. David Temperley used a MIDI- and music theory. based approach to implement many components of the theory [9] but has not yet treated the topology of the space. The Mathematical Music Theory Group at the Techni- 1. -

Towards Automatic Extraction of Harmony Information from Music Signals
Towards Automatic Extraction of Harmony Information from Music Signals Thesis submitted in partial fulfilment of the requirements of the University of London for the Degree of Doctor of Philosophy Christopher Harte August 2010 Department of Electronic Engineering, Queen Mary, University of London I certify that this thesis, and the research to which it refers, are the product of my own work, and that any ideas or quotations from the work of other people, published or otherwise, are fully acknowledged in accordance with the standard referencing practices of the discipline. I acknowledge the helpful guidance and support of my supervisor, Professor Mark Sandler. 2 Abstract In this thesis we address the subject of automatic extraction of harmony information from audio recordings. We focus on chord symbol recognition and methods for evaluating algorithms designed to perform that task. We present a novel six-dimensional model for equal tempered pitch space based on concepts from neo-Riemannian music theory. This model is employed as the basis of a harmonic change detection function which we use to improve the performance of a chord recognition algorithm. We develop a machine readable text syntax for chord symbols and present a hand labelled chord transcription collection of 180 Beatles songs annotated using this syntax. This collection has been made publicly avail- able and is already widely used for evaluation purposes in the research community. We also introduce methods for comparing chord symbols which we subsequently use for analysing the statistics of the transcription collection. To ensure that researchers are able to use our transcriptions with confidence, we demonstrate a novel alignment algorithm based on simple audio fingerprints that allows local copies of the Beatles audio files to be accurately aligned to our transcriptions automatically. -

Musical Tension Curves and Its Applications
Musical Tension Curves and its Applications Min-Joon Yoo and In-Kwon Lee Department of Computer Science, Yonsei University [email protected], [email protected] Abstract Madson and Fredrickson 1993; Krumhansl 1996). In these attempts, the tension at each part of music has been mea- We suggest a graphical representation of the musical tension sured by manual inputs of the participants in the experiments. flow in tonal music using a piecewise parametric curve, which In our work, we developed several methods to automatically is a function of time illustrating the changing degree of ten- compute the tension of a chord by combining known experi- sion in a corresponding chord progression. The tension curve mental results. Then the series of tension values correspond- can be edited by using conventional curve editing techniques ing to the given chord progression in the input music are in- to reharmonize the original music with reflecting the user’s terpolated to construct a smooth piecewise parametric curve. demand to control the tension of music. We introduce three We exploit the B-spline curve representation that is one of the different methods to measure the tension of a chord in terms most suitable method to model a complex shape such as ten- of a specific key, which can be used to represent the tension sion flow. Once the tension curve is constructed, we can edit of the chord numerically. Then, by interpolating the series of the tension flow of the original music by geometrically edit- numerical tension values, a tension curve is constructed. In ing the tension curve, where the tension curve editing also this paper, we show the tension curve editing method can be generates the new reharmonization of the original chord pro- effectively used in several interesting applications: enhanc- gression. -

A Unified Helical Structure in 3D Pitch Space for Mapping Flat Musical Isomorphism
HEXAGONAL CYLINDRICAL LATTICES: A UNIFIED HELICAL STRUCTURE IN 3D PITCH SPACE FOR MAPPING FLAT MUSICAL ISOMORPHISM A Thesis Submitted to the Faculty of Graduate Studies and Research In Partial Fulfillment of the Requirements for the Degree of Master of Science in Computer Science University of Regina By Hanlin Hu Regina, Saskatchewan December 2015 Copytright c 2016: Hanlin Hu UNIVERSITY OF REGINA FACULTY OF GRADUATE STUDIES AND RESEARCH SUPERVISORY AND EXAMINING COMMITTEE Hanlin Hu, candidate for the degree of Master of Science in Computer Science, has presented a thesis titled, Hexagonal Cylindrical Lattices: A Unified Helical Structure in 3D Pitch Space for Mapping Flat Musical Isomorphism, in an oral examination held on December 8, 2015. The following committee members have found the thesis acceptable in form and content, and that the candidate demonstrated satisfactory knowledge of the subject material. External Examiner: Dr. Dominic Gregorio, Department of Music Supervisor: Dr. David Gerhard, Department of Computer Science Committee Member: Dr. Yiyu Yao, Department of Computer Science Committee Member: Dr. Xue-Dong Yang, Department of Computer Science Chair of Defense: Dr. Allen Herman, Department of Mathematics & Statistics Abstract An isomorphic keyboard layout is an arrangement of notes of a scale such that any musical construct has the same shape regardless of the root note. The mathematics of some specific isomorphisms have been explored since the 1700s, however, only recently has a general theory of isomorphisms been developed such that any set of musical intervals can be used to generate a valid layout. These layouts have been implemented in the design of electronic musical instruments and software applications. -

The Geometry of Tonal Space
BRIDGES Mathematical Connections in Art, Mnsic, and Science Hearing With Our Eyes: The Geometry of Tonal Space Julian L. Hook School of Music Penn State University University Park, PA 16802-1901 E-mail: [email protected] There is no intrinsic reason why musical structures should be represented graphically. Music is, after aU, an auditory phenomenon. It consists of vibrations transmitted through the air as sound waves, received by our ears and processed by our brains as acoustic data. Visual aspects of music-the arrangement of musicians on a stage or of notes on a page, the gyrations of the conductor, the shine of the piano-are of secondary importance in our understanding and appreciation of a musical work. Most human beings, however, are visually oriented. We rely on our eyes more than our ears or any of our other sense organs in finding our way around, identifying other people and objects, and learning new information. Because of this dependence on the visual world, most people find abstract concepts easier to grasp if they can somehow be visualized: if some sort of graphical, geometric representation can be devised showing, if only metaphorically, the important elements of the conceptual framework and their relationships with each other. Even if these elements exist in sound and time rather than in light and space, such a representation may help us to get our bearings and to interpret what we hear. Pitches, chords, and key areas are examples of musical elements that lend themselves well to graphical depiction. Many music theorists through the centuries have drawn diagrams showing the appearance of "tonal space" from various perspectives. -

A Combined Model of Sensory and Cognitive Representations Underlying Tonal Expectations in Music
Collins et al in press, Psychological Review August 9, 2013 TITLE: A combined model of sensory and cognitive representations underlying tonal expectations in music: from audio signals to behavior AUTHORS: Tom Collins*‡, Barbara Tillmann#, Frederick S. Barrett*†, Charles Delbé#, and Petr Janata*† AFFILIATIONS: *Center for Mind and Brain, University of California, Davis, USA ‡Department of Computational Perception, Johannes Kepler University, Linz, Austria #Lyon Neuroscience Research Center, Lyon, France †Department of Psychology, University of California, Davis, USA RUNNING HEAD: sensory and cognitive contributions to tonal expectations KEYWORDS: tonality, torus, priming, music information retrieval, pitch CODE AND DATA: http://atonal.ucdavis.edu/resources/software/jlmt/tonmodcomp/ CORRESPONDENCE: Petr Janata Center for Mind and Brain 267 Cousteau Place Davis, CA 95618 phone: 530-297-4471 fax: 530-297-4400 e-mail: [email protected] Collins et al in press, Psychological Review August 9, 2013 ABSTRACT Listeners’ expectations for melodies and harmonies in tonal music are perhaps the most studied aspect of music cognition. Long debated has been whether faster response times (RTs) to more strongly primed events (in a music theoretic sense) are driven by sensory or cognitive mechanisms, such as repetition of sensory information or activation of cognitive schemata that reflect learned tonal knowledge, respectively. We analyzed over 300 stimuli from seven priming experiments comprising a broad range of musical material, using a model that transforms raw audio signals through a series of plausible physiological and psychological representations spanning a sensory–cognitive continuum. We show that RTs are modeled, in part, by information in periodicity pitch distributions, chroma vectors, and activations of tonal space – a representation on a toroidal surface of the major/minor key relationships in Western tonal music.