Status Review, Disease Risk Analysis and Conservation Action Plan for The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Geographic Variation in the Matamata Turtle, Chelus Fimbriatus, with Observations on Its Shell Morphology and Morphometry
n*entilkilt ilil Biok,gr', 1995. l(-l):19: 1995 by CheloninD Research Foundltion Geographic Variation in the Matamata Turtle, Chelus fimbriatus, with Observations on its Shell Morphology and Morphometry MlncBLo R. SANcnnz-Vu,urcnAr, PnrER C.H. PnrrcHARD:, ArrnEro P.rorrLLo-r, aNn Onan J. LINlnBs3 tDepartment of Biological Anthropolog-,- and Anatomy, Duke lJniversin' Medical Cetter. Box 3170, Dtu'hcun, North Carolina277l0 USA IFat 919-684-8034]; 2Florida Audubotr Societ-t, 460 High,n;a,- 436, Suite 200, Casselberry, Florida 32707 USA: iDepartanento de Esttdios Anbientales, llniyersitlad Sinzrin Bolltnt", Caracas ]O80-A, APDO 89OOO l/enerte\a Ansrucr. - A sample of 126 specimens of Chelusftmbriatus was examined for geographic variation and morphology of the shell. A high degree of variation was found in the plastral formula and in the shape and size of the intergular scute. This study suggests that the Amazon population of matamatas is different from the Orinoco population in the following characters: shape ofthe carapace, plastral pigmentation, and coloration on the underside of the neck. Additionatly, a preliminary analysis indicates that the two populations could be separated on the basis of the allometric growth of the carapace in relation to the plastron. Kry Wonus. - Reptilia; Testudinesl Chelidae; Chelus fimbriatus; turtle; geographic variationl allometryl sexual dimorphism; morphology; morphometryl osteology; South America 'Ihe matamata turtle (Chelus fimbricttus) inhabits the scute morpholo..ey. Measured characters (in all cases straight- Amazon, Oyapoque. Essequibo. and Orinoco river systems line) were: maximum carapace len.-uth (CL). cArapace width of northern South America (Iverson. 1986). Despite a mod- at the ler,'el of the sixth marginal scute (CW). -

Hollow-Bearing Trees As a Habitat Resource Along an Urbanisation Gradient
Hollow-Bearing Trees as a Habitat Resource along an Urbanisation Gradient Author Treby, Donna Louise Published 2014 Thesis Type Thesis (PhD Doctorate) School Griffith School of Environment DOI https://doi.org/10.25904/1912/1674 Copyright Statement The author owns the copyright in this thesis, unless stated otherwise. Downloaded from http://hdl.handle.net/10072/367782 Griffith Research Online https://research-repository.griffith.edu.au Hollow-bearing Trees as a Habitat Resource along an Urbanisation Gradient Donna Louise Treby MPhil (The University of Queensland) Environmental Futures Centre. Griffith School of Environment, Griffith University, Gold Coast. A thesis submitted for the fulfilment for the requirements of the degree of Doctor of Philosophy. December 2013. “If we all did the things we are capable of doing. We would literally live outstanding lives. I think; if we all lived our lives this way, we would truly create an amazing world.” Thomas Edison. i Acknowledgements: It would be remiss of me if I did not begin by acknowledging my principal supervisor Dr Guy Castley, for the inception, development and assistance with the completion of this study. Your generosity, open door policy and smiling face made it a pleasure to work with you. I owe you so much, but all I can give you is my respect and heartfelt thanks. Along with my associate supervisor Prof. Jean-Marc Hero their joint efforts inspired me and opened my mind to the complexities and vagaries of ecological systems and processes on such a large scale. To my volunteers in the field, Katie Robertson who gave so much of her time and help in the early stages of my project, Agustina Barros, Ivan Gregorian, Sally Healy, Guy Castley, Katrin Lowe, Kieran Treby, Phil Treby, Erin Wallace, Nicole Glenane, Nick Clark, Mark Ballantyne, Chris Tuohy, Ryan Pearson and Nickolas Rakatopare all contributed to the collection of data for this study. -

Download Vol. 33, No. 3
1. , F -6 ~.: 1/JJ/im--3'PT* JL* iLLLZW 1- : s . &, , I ' 4% Or *-* 0 4 Z 0 8 of the FLORIDA STATE MUSEUM Biological Sciences Volume 33 1988 Number 3 REPRODUCTIVE STRATEGIES OF SYMPATRIC FRESHWATER EMYDID TURTLES IN NORTHERN PENINSULAR FLORIDA Dale R. Jackson 3-C p . i ... h¢ 4 f .6/ I Se 4 .¢,$ I - - 64». 4 +Ay. 9.H>« UNIVERSITY OF FLORIDA GAINESVILLE Numbers of the BULLETIN OF TIIE FLORIDA STATE MUSEUM, BIOLOGICAL SCIENCES, are published at irregular intervals. Volumes contain about 300 pages and,are not necessarily completed in any one calendar year. S. DAVID WEBB, Editor OLIVER L. AUSTIN, JR., Editor Bile,ints RHODA J. BRYANT, Managing Editor Communications concerning purchase or exchange of the publications and all manuscripts should be addressed to: Managing Editor, Bulletin; Florida State Museum; University of Florida; Gainesville FL 32611; U.S.A. This public document was promulgated at an annual cost of $2003.53 or $2.000 per copy. It makes available to libraries, scholars, and all interested persons the results of researches in the natural sciences, emphasizing the circum- Caribbean region. ISSN: 0071-6154 CODEN: BFSBAS Publication date: 8/27 Price: $2.00 REPRODUCTIVE STRATEGIES OF SYMPATRIC FRESHWATER EMYDID TURTLES IN NORTHERN PENINSULAR FLORIDA Dale R. Jackson Frontispiece. Alligator nest on Payne's Prairie, Alachua County, Florida, opened to expose seven clutches of Psmdenzys nelsoni eggs and one clutch of Trioi,br ferox eggs (far lower right) surrounding the central clutch of alligator eggs. Most of the alligator eggs had been destroyed earlier by raccoons. REPRODUCTIVE STRATEGIES OF SYMPATRIC FRESHWATER EMYDID TURTLES IN NORTHERN PENINSULAR FLORIDA Dale R. -
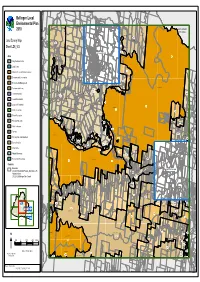
LEP 2010 LZN Template
WILD CATTLE CREEK E1E1E1 DORRIGO RU2RU2 GLENREAGH MULDIVA RU2RU2 OLD BILLINGS RD RAILWAY NATURE COAST TYRINGHAM RESERVE RD RD Bellingen Local E1EE1E11 BORRA CREEK DORRIGO NATIONAL PARK CORAMBA RD E3EE3E33 SLINGSBYS Environmental Plan LITTLE MURRAY RIVER RU2RRU2U2 RD LITTLE PLAIN CREEK BORRA CREEK TYRINGHAM COFFSCOFFS HARBOURHARBOUR 2010 RD CITYCCITYITY COUNCILCOUNCIL BREAKWELLS WILD CATTLE CREEK RD RU2RRU2U2 CORAMBA RD RU2RU2 NEAVES NEAVES RD RD SLINGSBYS RD Land Zoning Map LITTLE MURRAY RIVER OLD COAST RD E3EE3E33 RU2RRU2U2 Sheet LZN_004 E3EE3E33 DEER VALE RD OLD CORAMBA TYRINGHAM RD NTH RD ROCKY CREEK RU1RU1 BARTLETTS RD ReferRefer toto mapmap LZN_004ALZN_004A Zone RU1RRU1U1 BIELSDOWN RIVER E1EE1E11 DEER VALE RD B1 Neighbourhood Centre RU1RU1 DORRIGO NATIONAL PARK B2 Local Centre JOHNSENS WATERFALL WAY OLD CORAMBA WATERFALL WAY RD STH EE3E3E33 E1 National Parks and Nature Reserves E3E3E3 BENNETTS RD E2 Environmental Conservation WATERFALL WAY SHEPHERDS RD RU1RRU1U1 E3 Environmental Management DOME RD EVERINGHAMS ROCKY CREEK RD BIELSDOWN RIVER DORRIGO NATIONAL PARK E4 Environmental Living E3EE3E33 SP1SP1SP1 E3E3E3 SHEPHERDS RD WHISKY CEMETERYCCEMETERYEMETERY DOME RD IN1 General Industrial CREEK RD RU1RRU1U1 RU1RRU1U1 WOODLANDS RD WATERFALL R1 General Residential WAY RU1RRU1U1 PROMISED RU1RU1 E3E3E3 LAND RD WATERFALL WAY R5 Large Lot Residential WHISKY CREEK E1EE1E11 RU1RU1 DORRIGO NATIONAL PARK E1E1E1 E1EE1E11 RE1 Public Recreation BIELSDOWN RIVER E3EE3E33 ROCKY CREEK RD RE2 Private Recreation WHISKY CREEK RD RU1RRU1U1 RU2RU2 E3E3E3 -

Movement and Habitat Use of Australias Largest Snakenecked Turtle
bs_bs_bannerJournal of Zoology Journal of Zoology. Print ISSN 0952-8369 Movement and habitat use of Australia’s largest snake-necked turtle: implications for water management D. S. Bower1*, M. Hutchinson2 & A. Georges1 1 Institute for Applied Ecology, University of Canberra, Canberra, ACT, Australia 2 South Australian Museum, Adelaide, SA, Australia Keywords Abstract freshwater; radio-telemetry; home range; weir; tortoise; river; sex differences. Hydrological regimes strongly influence ecological processes in river basins. Yet, the impacts of management regimes are unknown for many freshwater taxa in Correspondence highly regulated rivers. We used radio-telemetry to monitor the movement and Deborah S. Bower, School of Environmental activity of broad-shelled river turtles Chelodina expansa to infer the impact of and Life Sciences, University of Newcastle, current water management practices on turtles in Australia’s most regulated river Callaghan, NSW 2308, Australia. – the Murray River. We radio-tracked C. expansa to (1) measure the range span Tel: +61 02 49212045; and examine the effect of sex, size and habitat type on turtle movement, and (2) Fax: +61 02 49 216923 examine habitat use within the river channel and its associated backwaters. C. Email: [email protected] expansa occupied all macro habitats in the river (main channel, backwater, swamp and connecting inlets). Within these habitats, females occupied discrete home *Present address: School of Environmental ranges, whereas males moved up to 25 km. The extensive movement of male and Life Sciences, University of Newcastle, turtles suggests that weirs and other aquatic barriers may interfere with movement Callaghan, NSW, 2308, Australia. and dispersal. Turtles regularly move between backwaters and the main river channel, which highlights the likely disturbance from backwater detachment, a Editor: Virginia Hayssen water saving practice in the lower Murray River. -

Long Swamp Fish and Frog Baseline Survey 2012
AQUASAVE CONSULTANTS Ecology, Monitoring and Conservation A registered business of: Regional, Focussed, On-ground Long Swamp Fish and Frog Baseline Survey 2012 A report to t he Glenelg Hopkins CMA By: Mark Bachmann, Nick Whiterod and Lachlan Farrington January 2013 Aquasave - NGT: Long Swamp Fish and Frog Baseline Survey 2012 This report may be cited as: Bachmann, M., Whiterod, N. & Farrington, L. (2013) Long Swamp Fish and Frog Baseline Survey 2012. A report to the Glenelg Hopkins CMA. Aquasave Consultants – Nature Glenelg Trust. Table of Contents EXECUTIVE SUMMARY .......................................................................................................................... 4 1. Introduction ...................................................................................................................................... 5 2. Site Locations and Sampling Methods .............................................................................................. 5 2.1 Native Fish ........................................................................................................................... 5 2.2 Frogs .................................................................................................................................... 9 3. Results............................................................................................................................................. 11 3.1 Native Fish ........................................................................................................................ -

Gwydir Waterbird and Fish Habitat Study: Final Reportdownload
Gwydir Waterbird and Fish Habitat Study Final report Cover photographs Front page: Boyanga Waterhole, Gingham Watercourse (Credit: J. Spencer); Golden perch, Mehi River (Credit: J. Spencer); Great egret (Credit: M. Carpenter). Citation This report can be cited as follows: Spencer, J.A., Heagney, E.C. and Porter, J.L. 2010. Final report on the Gwydir waterbird and fish habitat study. NSW Wetland Recovery Program. Rivers and Wetlands Unit, Department of Environment, Climate Change and Water NSW and University of New South Wales, Sydney. Acknowledgments Funding for this project was provided by the NSW Government and the Australian Government’s Water for the Future – Water Smart Australia program. Richard Allman, Sharon Bowen, Kirsty Brennan, Ben Daly, Simon Hunter, Jordan Iles, Jeff Kelleway, Lisa Knowles, Yoshi Kobayashi, Natalia Perera, Shannon Simpson, Rachael Thomas (DECCW), Neal Foster (NOW), Gus Porter and Ainslee Lions provided field support. Thanks to our pilots Richard Byrne (DECCW) and James Rainger (Fleet Helicopters) for their assistance with aerial waterbird surveys. Daryl Albertson (DECCW), Neal Foster, Liz Savage and James Houlahan (Border Rivers–Gwydir CMA) and Glenn Wilson (UNE) gave advice on field site locations. Jennifer and Bruce Southeron (Old Dromana), Sam Kirkby (Bunnor), Howard Blackburn (Crinolyn), Bill Johnson (DECCW), Liz Savage (Border Rivers–Gwydir CMA), Neal Foster, Tara Schalk (NOW), Richard Ping Kee (Moree fishing club), Dick Cooper, Greg Clancy and the NSW Bird Atlassers contributed historical information on waterbird and fish populations in the Gwydir. Sue Powell (ANU) supplied information on the flooding history and flow data for the Gwydir Wetlands. Mark McGrouther and Sally Reader (Australian Museum, Sydney) assisted with the identification of fish species. -

THE BIDGEE BULLETIN Quarterly Newsletter of the Murrumbidgee Monitoring Program
M A R C H 2 0 2 0 I S S U E 3 THE BIDGEE BULLETIN Quarterly Newsletter of the Murrumbidgee Monitoring Program WATERING OUTCOMES Welcome to Issue 3 of The Bidgee Bulletin. The field monitoring season is now complete, with As in previous years Commonwealth environmental water the last of the four wetland surveys conducted is being used to support aquatic plants and animals in the over the last two weeks of March. In this issue Murrumbidgee Selected Area. This year environmental we review the highlights of the season and water was largely used to target floodplains and wetlands summarise the outcomes from Commonwealth to improve water quality, support populations of water environmental watering actions during the 2019- dependent plants and animals, maintain frog populations 20 water year. We also introduce our Chief and create breeding opportunities for threatened species Twitcher from the NSW DPIE, Dr Jennifer including the southern bell frog and Australasian bittern. Spencer. Continued dry conditions in Spring 2019 meant that The Bidgee Bulletin is a quarterly newsletter environmental water needed to be carefully managed and designed to provide updates on our progress as focused on high priority outcomes. These included we monitor the ecological outcomes of maintaining critical refuge habitats - Wagourah Lagoon, Commonwealth environmental water flows in the Yarradda Lagoon, Telephone Creek and Tala Creek. Murrumbidgee Selected Area. The 2019-2022 Maintenance of these wetland habitats is important for program builds on the previous five year native fish and turtles, and the Murrumbidgee refuge sites monitoring period (2014-2019) and uses many continue to support high native fish diversity with large of the same methods. -

Demographic Consequences of Superabundance in Krefft's River
i The comparative ecology of Krefft’s River Turtle Emydura krefftii in Tropical North Queensland. By Dane F. Trembath B.Sc. (Zoology) Applied Ecology Research Group University of Canberra ACT, 2601 Australia A thesis submitted in fulfilment of the requirements of the degree of Masters of Applied Science (Resource Management). August 2005. ii Abstract An ecological study was undertaken on four populations of Krefft’s River Turtle Emydura krefftii inhabiting the Townsville Area of Tropical North Queensland. Two sites were located in the Ross River, which runs through the urban areas of Townsville, and two sites were in rural areas at Alligator Creek and Stuart Creek (known as the Townsville Creeks). Earlier studies of the populations in Ross River had determined that the turtles existed at an exceptionally high density, that is, they were superabundant, and so the Townsville Creek sites were chosen as low abundance sites for comparison. The first aim of this study was to determine if there had been any demographic consequences caused by the abundance of turtle populations of the Ross River. Secondly, the project aimed to determine if the impoundments in the Ross River had affected the freshwater turtle fauna. Specifically this study aimed to determine if there were any difference between the growth, size at maturity, sexual dimorphism, size distribution, and diet of Emydura krefftii inhabiting two very different populations. A mark-recapture program estimated the turtle population sizes at between 490 and 5350 turtles per hectare. Most populations exhibited a predominant female sex-bias over the sampling period. Growth rates were rapid in juveniles but slowed once sexual maturity was attained; in males, growth basically stopped at maturity, but in females, growth continued post-maturity, although at a slower rate. -

Bellinger and Kalang River Estuaries Erosion Study
Bellinger and Kalang River Estuaries Erosion Study Damon Telfer GECO Environmental Tim Cohen IRM Consultants February 2010 Report prep ared for BELLINGEN SHIRE COUNCIL DISCLAIMER Whilst every care has been taken in compiling this erosion study and report, no assurances are given that it is free from error or omission. The authors make no expressed or implied warranty of the accuracy or fitness of the recommendations in the report and disclaim all liability for the consequences of anything done or omitted to be done by any person in reliance upon these recommendations. The use of this report is at the user’s risk and discretion. The authors will not be liable for any loss, damage, costs or injury including consequential, incidental, or financial loss arising out of the use of this report. This report was produced with financial assistance from the NSW Government through the Department of Environment, Climate Change and Water. This document does not necessarily represent the opinions of the NSW Government or the Department of Environment, Climate Change and Water. Damon Telfer GECO Environmental 5 Arcadia Lane, GRASSY HEAD NSW 2441 Email: [email protected] © Damon Telfer, February 2010. This document is copyright and cannot be reproduced in part or whole without the express permission of the author. A permanent, irrevocable royalty-free, non-exclusive license to make these reports, documents and any other materials publicly available and to otherwise communicate, reproduce, adapt and publicise them on a non- profit basis is granted by the authors to Bellingen Shire Council and the Department of Environment, Climate Change and Water. -

Eastern Snake-Necked Turtle
Husbandry Manual for Eastern Snake-Necked Turtle Chelodina longicollis Reptilia: Chelidae Image Courtesy of Jacki Salkeld Author: Brendan Mark Host Date of Preparation: 04/06/06 Western Sydney Institute of TAFE - Richmond Course Name and Number: 1068 Certificate 3 - Captive Animals Lecturers: Graeme Phipps/Andrew Titmuss/ Jacki Salkeld CONTENTS 1. Introduction 4 2. Taxonomy 5 2.1 Nomenclature 5 2.2 Subspecies 5 2.3 Synonyms 5 2.4 Other Common Names 5 3. Natural History 6 3.1 Morphometrics 6 3.1.1 Mass and Basic Body Measurements 6 3.1.2 Sexual Dimorphism 6 3.1.3 Distinguishing Features 7 3.2 Distribution and Habitat 7 3.3 Conservation Status 8 3.4 Diet in the Wild 8 3.5 Longevity 8 3.5.1 In the Wild 8 3.5.2 In Captivity 8 3.5.3 Techniques Used to Determine Age in Adults 9 4. Housing Requirements 10 4.1 Exhibit/Enclosure Design 10 4.2 Holding Area Design 10 4.3 Spatial Requirements 11 4.4 Position of Enclosures 11 4.5 Weather Protection 11 4.6 Temperature Requirements 12 4.7 Substrate 12 4.8 Nestboxes and/or Bedding Material 12 4.9 Enclosure Furnishings 12 5. General Husbandry 13 5.1 Hygiene and Cleaning 13 5.2 Record Keeping 13 5.3 Methods of Identification 13 5.4 Routine Data Collection 13 6. Feeding Requirements 14 6.1 Captive Diet 14 6.2 Supplements 15 6.3 Presentation of Food 15 1 7. Handling and Transport 16 7.1 Timing of Capture and Handling 16 7.2 Capture and Restraint Techniques 16 7.3 Weighing and Examination 17 7.4 Release 17 7.5 Transport Requirements 18 7.5.1 Box Design 18 7.5.2 Furnishings 19 7.5.3 Water and Food 19 7.5.4 Animals Per Box 19 7.5.5 Timing of Transportation 19 7.5.6 Release from Box 19 8. -

Testudines: Chelidae) of Australia, New Guinea and Indonesia
Zoological Journal of the Linnean Society, 2002, 134, 401–421. With 7 figures Electrophoretic delineation of species boundaries within the genus Chelodina (Testudines: Chelidae) of Australia, New Guinea and Indonesia ARTHUR GEORGES1*, MARK ADAMS2 and WILLIAM McCORD3 1Applied Ecology Research Group, University of Canberra, ACT 2601, Australia 2Evolutionary Biology Unit, South Australian Museum, North Terrace, Adelaide, SA 5001, Australia 3East Fishkill Animal Hospital, 285 Rt 82, Hopewell Junction NY 12533, USA Received February 2001; revised and accepted for publication June 2001 A total of 281 specimens of long-necked chelid turtles (Chelodina) were obtained from drainages of Australia, Papua New Guinea and the island of Roti in Indonesia. Ten diagnosable taxa were identified using allozyme profiles at 45 presumptive loci. Chelodina expansa, C. parkeri, C. rugosa and C. burrungandjii are in a Group A clade, C. longi- collis, C. novaeguineae, C. steindachneri, C. pritchardi and C. mccordi are in a Group B clade, and C. oblonga is in a monotypic Group C clade, with each clade thought to represent a distinct subgenus. Chelodina siebenrocki is syn- onymised with C. rugosa. An eleventh taxon, C. reimanni, could not be distinguished from C. novaeguineae on the basis of allozyme profiles, but it is morphologically distinct. Its status is therefore worthy of further investigation. Three instances of natural hybridization were detected. Chelodina rugosa and C. novaeguineae hybridize in the Gulf country of Queensland, with evidence of backcrossing to C. novaeguineae. Chelodina longicollis and C. novaeguineae hybridize in central coastal Queensland, and C. rugosa and C. burrungandjii hybridize along their zone of contact in the plateau escarpment streams and pools.