Increasing Phylogenetic Stochasticity at High Elevations on Summits Across A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
High Resolution Adobe PDF
115°20'0"W 115°0'0"W 114°40'0"W 114°20'0"W PISTOL LAKE " CHINOOK MOUNTAIN ARTILLERY DOME SLIDEROCK RIDGE FALCONBERRY PEAK ROCK CREEK SHELDON PEAK Red Butte "Grouse Creek Peak WHITE GOAWTh iMte OVaUlleNyT MAoIuNntain LITTLE SOLDIER MOUNTAIN N FD " N FD 6 8 8 T d Parker Mountain 6 Greyhound Mountain r R a k i e " " 5 2 l e 0 1 0 r 0 0 il 1 C l i a 1 n r o Big Soldier Mountain a o e pi r n Morehead Mountain T Pinyon Peak L White MoSunletain g Deer Rd " T " HONEYMOON LAKE " " BIG SOLDIER MOUNTAIN SOLDIER CREEK GREYHOUND MOUNTAIN PINYON PEAK CASTO SHERMAN PEAK CHALLIS CREEK LAKES TWIN PEAKS PATS CREEK Lo FRANK CHURCH - RIVER OF NO RETURN WILDERNESS o n Sherman Peak C Mayfield Peak Corkscrew Mountain r " d e " " R ek ls R l d a Mosquito Flat Reservoir F r e Langer Peak rl g T g k a Ruffneck Peak " ac d D P R d " k R Blue Bunch Mo"untain d e M e k R ill C r e Bear Valley Mountain k e e htmile r " e ig C r E C en r C re d ave Estes Mountain e G ar B e k " R BLUE BUNCH MOUNTAIN d CAPE HORN LAKES LANGER PEAK KNAPP LAKES MOUNT JORDAN l Forest CUSTER ELEVENMILE CREEK BAYHORRSaEm sLhAorKn EMountaiBn AYHORSE Nat De Rd Keysto"ne Mountain velop Road 579 d R " Cabin Creek Peak Red Mountain rk Cape Horn MounCtaaipne Horn Lake #1 o Bay d " Bald Mountain F hors R " " e e Cr 2 d e eek 8 R " nk Rd 5 in Ya d a a nt o ou Lucky B R S A L M O N - C H A L L I S N Fo S p M y o 1 C d Bachelor Mountain R q l " u e 2 5 a e d v y 19 p R Bonanza Peak a B"ald Mountain e d e w Nf 045 D w R R N t " s H s H C d " e sf r e o Basin Butte r 0 t U ' o r e F a n e 0 l t 21 t -

Mcclinton Unr 0139M 13052.Pdf
University of Nevada, Reno Habitat preferences, intraspecific variation, and restoration of a rare soil specialist in northern Nevada A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Natural Resources and Environmental Science by Jamey D. McClinton Dr. Elizabeth A. Leger/Thesis Advisor December, 2019 Copyright by Jamey D. McClinton 2019 All Rights Reserved We recommend that the thesis prepared under our supervision by Jamey D. McClinton Entitled Habitat preferences, intraspecific variation, and restoration of a rare soil specialist in northern Nevada be accepted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Elizabeth Leger, Ph.D., Advisor Paul Verburg, Ph.D., Committee member Thomas Parchman, Ph.D., Graduate School Representative David W. Zeh, Ph.D., Dean, Graduate School December-2019 i Abstract Edaphic specialization in plants is associated with the development of novel adaptations that frequently lead to speciation, causing unique edaphic environments to be associated with rare and endemic plant species worldwide. These species contribute significantly to global biodiversity, but are especially vulnerable to disturbance and climate change because of their inherently patchy distributions and locally adapted populations. Successful conservation of these species depends upon understanding their habitat requirements and the amounts and distributions of genetic and phenotypic diversity among populations. Little is known about the habitat requirements or -

Sawtooth Interpretive & Historical Association
Sawtooth Interpretive & Historical Association GREG WEBBER A SPECIAL THANK YOU TO ALL OUR 2020 SUPPORTERS! Sawtooth Interpretive & Historical Association is honored to receive funding and in-kind contributions from individuals, foundations, and businesses that support our mission. We extend our sincerest thanks to our past and present members, donors and volunteers. PROGRAM PATRON $5,000+ Sari and Gary O’Malley Anonymous Jack Baird Melissa and David Pinney Kay Davies Jennifer Osborne Susannah Avey Sherrill and Ervine Baird Lynn Rosellini and David Whitman Sandy and Rich Ostrogorsky Dolores Bernardo Marsha and Bob Beckwith Leidy and Sadler Samson SAWTOOTH BENEFACTOR Carol Cole and Jim Rineholt Marilyn Burdwell Linda and Bill Bein Patty and Jack See $1,000+ Jim and Adrienne Stark Erica Cole Emmy Blechmann Art Selin In memory of Eleanor Mae Dixon Erik Storlie Rebecca Converse Joan and Mike Boren Rozalys Smith Ann and Paul Hill Spencer and Evelyn Strand Peggy Dean Marjorie and Robert Boren Michelle and Chris Stephens Idaho Rocky Mountain Ranch Wendy and Jeff Turner Gayle Dixon Kathy and Kent Browning Wendy and Jack Stevens Harvey Dale and Debra LaMorte Dan and Zella Unger Ellen and Tom Glaccum Terry and Hans Carstensen Phyllis and Fred Stewart The Obletz Family John and Sue Van Der Wal Lin Gray Mr. and Mrs. Harry J Chavanne Erik Storlie Nancy and Bob Warmack Harlan Hague Wei and Jon Christianson Anne and Tom Stuart SUSTAINING MEMBERS Mike and Colleen Werner Idaho Candy Company Stacey and Terry Clark Deanne Thompson $250+ Debbie and Stewart Wilder Dick and Mary Lou Kinney Audra and Jeff Clegg Christy and Charlie Thompson Leslie Benz Wolcott Family & Danner Log Cabins Fullmer Latter III Kathy and Steve Cole Dick Waite Family Kent Browning Patricia Young Melanie Lynn in honor of Anne and Steve Cunningham Dr. -

National Wetlands Inventory Map Report for Quinault Indian Nation
National Wetlands Inventory Map Report for Quinault Indian Nation Project ID(s): R01Y19P01: Quinault Indian Nation, fiscal year 2019 Project area The project area (Figure 1) is restricted to the Quinault Indian Nation, bounded by Grays Harbor Co. Jefferson Co. and the Olympic National Park. Appendix A: USGS 7.5-minute Quadrangles: Queets, Salmon River West, Salmon River East, Matheny Ridge, Tunnel Island, O’Took Prairie, Thimble Mountain, Lake Quinault West, Lake Quinault East, Taholah, Shale Slough, Macafee Hill, Stevens Creek, Moclips, Carlisle. • < 0. Figure 1. QIN NWI+ 2019 project area (red outline). Source Imagery: Citation: For all quads listed above: See Appendix A Citation Information: Originator: USDA-FSA-APFO Aerial Photography Field Office Publication Date: 2017 Publication place: Salt Lake City, Utah Title: Digital Orthoimagery Series of Washington Geospatial_Data_Presentation_Form: raster digital data Other_Citation_Details: 1-meter and 1-foot, Natural Color and NIR-False Color Collateral Data: . USGS 1:24,000 topographic quadrangles . USGS – NHD – National Hydrography Dataset . USGS Topographic maps, 2013 . QIN LiDAR DEM (3 meter) and synthetic stream layer, 2015 . Previous National Wetlands Inventories for the project area . Soil Surveys, All Hydric Soils: Weyerhaeuser soil survey 1976, NRCS soil survey 2013 . QIN WET tables, field photos, and site descriptions, 2016 to 2019, Janice Martin, and Greg Eide Inventory Method: Wetland identification and interpretation was done “heads-up” using ArcMap versions 10.6.1. US Fish & Wildlife Service (USFWS) National Wetlands Inventory (NWI) mapping contractors in Portland, Oregon completed the original aerial photo interpretation and wetland mapping. Primary authors: Nicholas Jones of SWCA Environmental Consulting. 100% Quality Control (QC) during the NWI mapping was provided by Michael Holscher of SWCA Environmental Consulting. -

National List of Vascular Plant Species That Occur in Wetlands 1996
National List of Vascular Plant Species that Occur in Wetlands: 1996 National Summary Indicator by Region and Subregion Scientific Name/ North North Central South Inter- National Subregion Northeast Southeast Central Plains Plains Plains Southwest mountain Northwest California Alaska Caribbean Hawaii Indicator Range Abies amabilis (Dougl. ex Loud.) Dougl. ex Forbes FACU FACU UPL UPL,FACU Abies balsamea (L.) P. Mill. FAC FACW FAC,FACW Abies concolor (Gord. & Glend.) Lindl. ex Hildebr. NI NI NI NI NI UPL UPL Abies fraseri (Pursh) Poir. FACU FACU FACU Abies grandis (Dougl. ex D. Don) Lindl. FACU-* NI FACU-* Abies lasiocarpa (Hook.) Nutt. NI NI FACU+ FACU- FACU FAC UPL UPL,FAC Abies magnifica A. Murr. NI UPL NI FACU UPL,FACU Abildgaardia ovata (Burm. f.) Kral FACW+ FAC+ FAC+,FACW+ Abutilon theophrasti Medik. UPL FACU- FACU- UPL UPL UPL UPL UPL NI NI UPL,FACU- Acacia choriophylla Benth. FAC* FAC* Acacia farnesiana (L.) Willd. FACU NI NI* NI NI FACU Acacia greggii Gray UPL UPL FACU FACU UPL,FACU Acacia macracantha Humb. & Bonpl. ex Willd. NI FAC FAC Acacia minuta ssp. minuta (M.E. Jones) Beauchamp FACU FACU Acaena exigua Gray OBL OBL Acalypha bisetosa Bertol. ex Spreng. FACW FACW Acalypha virginica L. FACU- FACU- FAC- FACU- FACU- FACU* FACU-,FAC- Acalypha virginica var. rhomboidea (Raf.) Cooperrider FACU- FAC- FACU FACU- FACU- FACU* FACU-,FAC- Acanthocereus tetragonus (L.) Humm. FAC* NI NI FAC* Acanthomintha ilicifolia (Gray) Gray FAC* FAC* Acanthus ebracteatus Vahl OBL OBL Acer circinatum Pursh FAC- FAC NI FAC-,FAC Acer glabrum Torr. FAC FAC FAC FACU FACU* FAC FACU FACU*,FAC Acer grandidentatum Nutt. -

Wilderness Study Areas
I ___- .-ll..l .“..l..““l.--..- I. _.^.___” _^.__.._._ - ._____.-.-.. ------ FEDERAL LAND M.ANAGEMENT Status and Uses of Wilderness Study Areas I 150156 RESTRICTED--Not to be released outside the General Accounting Wice unless specifically approved by the Office of Congressional Relations. ssBO4’8 RELEASED ---- ---. - (;Ao/li:( ‘I:I)-!L~-l~~lL - United States General Accounting OfTice GAO Washington, D.C. 20548 Resources, Community, and Economic Development Division B-262989 September 23,1993 The Honorable Bruce F. Vento Chairman, Subcommittee on National Parks, Forests, and Public Lands Committee on Natural Resources House of Representatives Dear Mr. Chairman: Concerned about alleged degradation of areas being considered for possible inclusion in the National Wilderness Preservation System (wilderness study areas), you requested that we provide you with information on the types and effects of activities in these study areas. As agreed with your office, we gathered information on areas managed by two agencies: the Department of the Interior’s Bureau of Land Management (BLN) and the Department of Agriculture’s Forest Service. Specifically, this report provides information on (1) legislative guidance and the agency policies governing wilderness study area management, (2) the various activities and uses occurring in the agencies’ study areas, (3) the ways these activities and uses affect the areas, and (4) agency actions to monitor and restrict these uses and to repair damage resulting from them. Appendixes I and II provide data on the number, acreage, and locations of wilderness study areas managed by BLM and the Forest Service, as well as data on the types of uses occurring in the areas. -

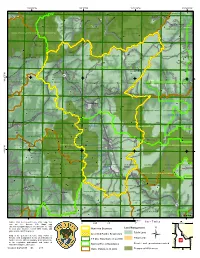
Little Wood River Management Area 5
Chapter III Little Wood River Management Area 5 eek Box Canyon Cr r e iv R d o o 1.2 W ig r B 4.2 e v rk i o R F t d s a o E o W le t t i !9 Federal Gulch L !9 Sawmill Pioneer Mountains IRA k e re C n o o ld u 6.1 M 6.1 3.1 Copper !9 1.2 Creek 1 3 4 02468Miles Legend Management Prescription Categories 1.2 Recommended Wilderness 3.1 Passive Restoration and Maintenance of Aquatic, Terrestrial, and Hydrologic Resources 4.2 Roaded Recreation 6.1 Restoration and Maintenance Emphasis within Shrubland and Grassland Landscapes ¯ Non-Forest System Lands Wild & Scenic River Classification The Forest Service uses the most current and complete Eligible Wild & Scienic Rivers: Wild Classification data available. GIS data and product accuracy may vary. Inventoried Roadless Areas (IRAs) Using GIS products for purposes other than those intended may yield inaccurate or misleading results. Map produced by: B.Geesey, Sawtooth NF, 09/2009 Management Area 05. Little Wood River Location Map III - 177 Chapter III Little Wood River Management Area 5 Management Area 5 Little Wood River MANAGEMENT AREA DESCRIPTION Management Prescriptions - Management Area 5 has the following management prescriptions (see map on preceding page for distribution of prescriptions). Percent of Management Prescription Category (MPC) Mgt. Area 1.2 – Recommended Wilderness 57 3.1 – Passive Restoration and Maintenance of Aquatic, Terrestrial & Hydrologic Resources 7 4.2 – Roaded Recreation Emphasis Trace 6.1 – Restoration and Maintenance Emphasis within Shrubland & Grassland Landscapes 36 General Location and Description - Management Area 5 is comprised of lands administered by the Sawtooth National Forest within the Little Wood River drainage east of Ketchum and Sun Valley, Idaho (see map, preceding page). -

Polygonaceae of Alberta
AN ILLUSTRATED KEY TO THE POLYGONACEAE OF ALBERTA Compiled and writen by Lorna Allen & Linda Kershaw April 2019 © Linda J. Kershaw & Lorna Allen This key was compiled using informaton primarily from Moss (1983), Douglas et. al. (1999) and the Flora North America Associaton (2005). Taxonomy follows VAS- CAN (Brouillet, 2015). The main references are listed at the end of the key. Please let us know if there are ways in which the kay can be improved. The 2015 S-ranks of rare species (S1; S1S2; S2; S2S3; SU, according to ACIMS, 2015) are noted in superscript (S1;S2;SU) afer the species names. For more details go to the ACIMS web site. Similarly, exotc species are followed by a superscript X, XX if noxious and XXX if prohibited noxious (X; XX; XXX) according to the Alberta Weed Control Act (2016). POLYGONACEAE Buckwheat Family 1a Key to Genera 01a Dwarf annual plants 1-4(10) cm tall; leaves paired or nearly so; tepals 3(4); stamens (1)3(5) .............Koenigia islandica S2 01b Plants not as above; tepals 4-5; stamens 3-8 ..................................02 02a Plants large, exotic, perennial herbs spreading by creeping rootstocks; fowering stems erect, hollow, 0.5-2(3) m tall; fowers with both ♂ and ♀ parts ............................03 02b Plants smaller, native or exotic, perennial or annual herbs, with or without creeping rootstocks; fowering stems usually <1 m tall; fowers either ♂ or ♀ (unisexual) or with both ♂ and ♀ parts .......................04 3a 03a Flowering stems forming dense colonies and with distinct joints (like bamboo -

Yukon Wild Buckwheat Eriogonum Flavum Var. Aquilinum
COSEWIC Assessment and Status Report on the Yukon Wild Buckwheat Eriogonum flavum var. aquilinum in Canada SPECIAL CONCERN 2017 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2017. COSEWIC assessment and status report on the Yukon Wild Buckwheat Eriogonum flavum var. aquilinum in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. x + 32 pp. (http://www.registrelep-sararegistry.gc.ca/default.asp?lang=en&n=24F7211B-1). Production note: COSEWIC would like to acknowledge Allan Harris and Robert Foster for writing the status report on Yukon Wild Buckwheat, Eriogonum flavum var. aquilinum, in Canada, prepared under contract with Environment and Climate Change Canada. This report was overseen and edited by Jana Vamosi, Co-chair of the COSEWIC Vascular Plants Specialist Subcommittee, with significant input from Bruce Bennett, former Co-chair of COSEWIC Vascular Plants and current COSEWIC Vascular Plants Specialist Subcommittee member. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment and Climate Change Canada Ottawa, ON K1A 0H3 Tel.: 819-938-4125 Fax: 819-938-3984 E-mail: [email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur L’ériogone du Nord (Eriogonum flavum var. aquilinum) au Canada. Cover illustration/photo: Yukon Wild Buckwheat — Photo credit: Allan Harris. Her Majesty the Queen in Right of Canada, 2017. Catalogue No. CW69-14/758-2018E-PDF ISBN 978-0-660-26676-3 COSEWIC Assessment Summary Assessment Summary – November 2017 Common name Yukon Wild Buckwheat Scientific name Eriogonum flavum var. -

Part 2 – Fruticose Species
Appendix 5.2-1 Vegetation Technical Appendix APPENDIX 5.2‐1 Vegetation Technical Appendix Contents Section Page Ecological Land Classification ............................................................................................................ A5.2‐1‐1 Geodatabase Development .............................................................................................. A5.2‐1‐1 Vegetation Community Mapping ..................................................................................... A5.2‐1‐1 Quality Assurance and Quality Control ............................................................................ A5.2‐1‐3 Limitations of Ecological Land Classification .................................................................... A5.2‐1‐3 Field Data Collection ......................................................................................................... A5.2‐1‐3 Supplementary Results ..................................................................................................... A5.2‐1‐4 Rare Vegetation Species and Rare Ecological Communities ........................................................... A5.2‐1‐10 Supplementary Desktop Results ..................................................................................... A5.2‐1‐10 Field Methods ................................................................................................................. A5.2‐1‐16 Supplementary Results ................................................................................................... A5.2‐1‐17 Weed Species -

Annotated Checklist of Vascular Flora, Bryce
National Park Service U.S. Department of the Interior Natural Resource Program Center Annotated Checklist of Vascular Flora Bryce Canyon National Park Natural Resource Technical Report NPS/NCPN/NRTR–2009/153 ON THE COVER Matted prickly-phlox (Leptodactylon caespitosum), Bryce Canyon National Park, Utah. Photograph by Walter Fertig. Annotated Checklist of Vascular Flora Bryce Canyon National Park Natural Resource Technical Report NPS/NCPN/NRTR–2009/153 Author Walter Fertig Moenave Botanical Consulting 1117 W. Grand Canyon Dr. Kanab, UT 84741 Sarah Topp Northern Colorado Plateau Network P.O. Box 848 Moab, UT 84532 Editing and Design Alice Wondrak Biel Northern Colorado Plateau Network P.O. Box 848 Moab, UT 84532 January 2009 U.S. Department of the Interior National Park Service Natural Resource Program Center Fort Collins, Colorado The Natural Resource Publication series addresses natural resource topics that are of interest and applicability to a broad readership in the National Park Service and to others in the management of natural resources, including the scientifi c community, the public, and the NPS conservation and environmental constituencies. Manuscripts are peer-reviewed to ensure that the information is scientifi cally credible, technically accurate, appropriately written for the intended audience, and is designed and published in a professional manner. The Natural Resource Technical Report series is used to disseminate the peer-reviewed results of scientifi c studies in the physical, biological, and social sciences for both the advancement of science and the achievement of the National Park Service’s mission. The reports provide contributors with a forum for displaying comprehensive data that are often deleted from journals because of page limitations. -

Current Tracking List
Nevada Division of Natural Heritage Department of Conservation and Natural Resources 901 S. Stewart Street, Suite 5002, Carson City, Nevada 89701-5245 voice: (775) 684-2900 | fax: (775) 684-2909 | web: heritage.nv.gov At-Risk Plant and Animal Tracking List July 2021 The Nevada Division of Natural Heritage (NDNH) A separate list, the Plant and Animal Watch List, systematically curates information on Nevada's contains taxa that could become at-risk in the future. endangered, threatened, sensitive, rare, and at-risk plants and animals providing the most comprehensive Taxa on the At-Risk Plant and Animal Tracking List are source of information on Nevada’s imperiled organized by taxonomic group, and presented biodiversity. alphabetically by scientific name within each group. Currently, there are 639 Tracking List taxa: 285 plants, Nevada's health and economic well-being depend 209 invertebrates, 65 fishes, 9 amphibians, 7 reptiles, upon its biodiversity and wise land stewardship. This 27 birds, and 37 mammals. challenge increases as population and land-use pressures continue to grow. Nevada is among the top Documentation of population status, locations, or 10 states for both the diversity and the vulnerability of other updates or corrections for any of the taxa on its living heritage. With early planning and responsible this list are always welcome. Literature citations with development, economic growth and our biological taxonomic revisions and descriptions of new taxa are resources can coexist. NDNH is a central source for also appreciated. The Nevada Native Species Site information critical to achieving this balance. Survey Report form is available on our website under Management priorities for the state’s imperiled the Submit Data tab and is the preferred format for biodiversity are continually assessed, providing submitting information to NDNH.