ZYTIGA with Caution in Patients with a ZYTIGA Safely and Effectively
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pharmacokinetic Interactions Between Herbal Medicines and Drugs: Their Mechanisms and Clinical Relevance
life Review Pharmacokinetic Interactions between Herbal Medicines and Drugs: Their Mechanisms and Clinical Relevance Laura Rombolà 1 , Damiana Scuteri 1,2 , Straface Marilisa 1, Chizuko Watanabe 3, Luigi Antonio Morrone 1, Giacinto Bagetta 1,2,* and Maria Tiziana Corasaniti 4 1 Preclinical and Translational Pharmacology, Department of Pharmacy, Health and Nutritional Sciences, Section of Preclinical and Translational Pharmacology, University of Calabria, 87036 Rende, Italy; [email protected] (L.R.); [email protected] (D.S.); [email protected] (S.M.); [email protected] (L.A.M.) 2 Pharmacotechnology Documentation and Transfer Unit, Preclinical and Translational Pharmacology, Department of Pharmacy, Health and Nutritional Sciences, University of Calabria, 87036 Rende, Italy 3 Department of Physiology and Anatomy, Tohoku Pharmaceutical University, 981-8558 Sendai, Japan; [email protected] 4 School of Hospital Pharmacy, University “Magna Graecia” of Catanzaro and Department of Health Sciences, University “Magna Graecia” of Catanzaro, 88100 Catanzaro, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-0984-493462 Received: 28 May 2020; Accepted: 30 June 2020; Published: 4 July 2020 Abstract: The therapeutic efficacy of a drug or its unexpected unwanted side effects may depend on the concurrent use of a medicinal plant. In particular, constituents in the medicinal plant extracts may influence drug bioavailability, metabolism and half-life, leading to drug toxicity or failure to obtain a therapeutic response. This narrative review focuses on clinical studies improving knowledge on the ability of selected herbal medicines to influence the pharmacokinetics of co-administered drugs. Moreover, in vitro studies are useful to anticipate potential herbal medicine-drug interactions. -

Information for the User ZYTIGA 500 Mg Film-Coated Tablets Abiraterone
Package leaflet: Information for the user ZYTIGA 500 mg film-coated tablets abiraterone acetate Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. - Keep this leaflet. You may need to read it again. - If you have any further questions, ask your doctor or pharmacist. - This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours. - If you get any side effects talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4. What is in this leaflet: 1. What ZYTIGA is and what it is used for 2. What you need to know before you take ZYTIGA 3. How to take ZYTIGA 4. Possible side effects 5. How to store ZYTIGA 6. Contents of the pack and other information 1. What ZYTIGA is and what it is used for ZYTIGA contains a medicine called abiraterone acetate. It is used to treat prostate cancer in adult men that has spread to other parts of the body. ZYTIGA stops your body from making testosterone; this can slow the growth of prostate cancer. When ZYTIGA is prescribed for the early stage of disease where it is still responding to hormone therapy, it is used with a treatment that lowers testosterone (androgen deprivation therapy ). When you take this medicine your doctor will also prescribe another medicine called prednisone or prednisolone. This is to lower your chances of getting high blood pressure, having too much water in your body (fluid retention), or having reduced levels of a chemical known as potassium in your blood. -

Abiraterone Acetate for Chemotherapy-Naive
Fan et al. BMC Urology (2018) 18:110 https://doi.org/10.1186/s12894-018-0416-6 RESEARCHARTICLE Open Access Abiraterone acetate for chemotherapy- naive metastatic castration-resistant prostate cancer: a single-centre prospective study of efficacy, safety, and prognostic factors Liancheng Fan†, Baijun Dong†, Chenfei Chi†, Yanqing Wang†, Yiming Gong†, Jianjun Sha, Jiahua Pan, Xun Shangguan, Yiran Huang, Lixin Zhou* and Wei Xue* Abstract Background: To evaluate the efficacy and safety of abiraterone acetate (AA) plus prednisone compared with prednisone alone in Asian patients with chemotherapy-naive metastatic castration-resistant prostate cancer (mCRPC), and to identify predictive factors. Methods: We reviewed the medical records of 60 patients with chemotherapy-naive mCRPC at Renji Hospital who were treated with AA plus prednisone (n = 43) or prednisone alone (n = 17). All patients were assessed for prostate- specific antigen (PSA) response, PSA progression-free survival (PSA PFS), radiographic progression-free survival (rPFS), and overall survival (OS). The ability of several parameters to predict PSA PFS, rPFS, and OS was studied. Results: The median follow-up time was 14.0 months (range 7.0–18.5 months), at which time 19 death events had been reported: 11 in the AA + prednisone group and 8 in the prednisone group. The AA + prednisone group had significantly longer median PSA PFS (10.3 vs 3.0 months, P < 0.001), rPFS (13.9 vs 3.9 months, P < 0.001), and OS (23. 3 vs 17.5 months, P = 0.016) than the prednisone-alone group. The most frequently reported grade 3 or 4 adverse event in both the AA + prednisone and prednisone-alone groups was elevated alanine aminotransferase level in 5 of 43 patients (11.6%) and 2 of 17 patients (11.8%), respectively. -

Indinavir Sulfate Capsule Merck & Co., Inc
CRIXIVAN - indinavir sulfate capsule Merck & Co., Inc. ---------- CRIXIVAN® (INDINAVIR SULFATE) CAPSULES DESCRIPTION CRIXIVAN1 (indinavir sulfate) is an inhibitor of the human immunodeficiency virus (HIV) protease. CRIXIVAN Capsules are formulated as a sulfate salt and are available for oral administration in strengths of 100, 200, 333, and 400 mg of indinavir (corresponding to 125, 250, 416.3, and 500 mg indinavir sulfate, respectively). Each capsule also contains the inactive ingredients anhydrous lactose and magnesium stearate. The capsule shell has the following inactive ingredients and dyes: gelatin, titanium dioxide, silicon dioxide and sodium lauryl sulfate. The chemical name for indinavir sulfate is [1(1S,2R),5(S)]-2,3,5-trideoxy-N-(2,3-dihydro-2-hydroxy-1H-inden-1-yl)-5-[2-[[(1,1 dimethylethyl)amino]carbonyl]-4-(3-pyridinylmethyl)-1-piperazinyl]-2-(phenylmethyl)-D-erythro-pentonamide sulfate (1:1) salt. Indinavir sulfate has the following structural formula: Indinavir sulfate is a white to off-white, hygroscopic, crystalline powder with the molecular formula C36H47N5O4• H2SO4 and a molecular weight of 711.88. It is very soluble in water and in methanol. 1 Registered trademark of MERCK & CO., Inc. COPYRIGHT © 1996, 1997, 1998, 1999, 2004 MERCK & CO., Inc. All rights reserved MICROBIOLOGY Mechanism of Action HIV-1 protease is an enzyme required for the proteolytic cleavage of the viral polyprotein precursors into the individual functional proteins found in infectious HIV-1. Indinavir binds to the protease active site and inhibits the activity of the enzyme. This inhibition prevents cleavage of the viral polyproteins resulting in the formation of immature non-infectious viral particles. -

PATIENT FACT SHEET (Deltasone)
Prednisone PATIENT FACT SHEET (Deltasone) Prednisone (Deltasone) is part of a potent class of inflammatory conditions, including redness, anti-inflammatory agents, known as corticosteroids, swelling and pain. Prednisone is used to treat which are used to control inflammation of the rheumatoid arthritis, lupus, vasculitis, and many joints and organs. It is often used to treat a variety of other inflammatory diseases. WHAT IS IT? Dosing of prednisone varies widely depending on tablets take effect about 6 hours after taking the the state of the disease being treated. Doses used dose. Prednisone stops working soon after stopping in rheumatoid arthritis are commonly 5-10mg daily, the medication. If you have been taking prednisone while doses needed in lupus and vasculitis are often regularly for longer than 2 weeks, do not stop it 80mg daily, or sometimes higher. Prednisone usually suddenly. Instead, you should discuss a tapering HOW TO achieves its effect within 1-2 hours. The delayed release schedule with your physician. TAKE IT Most side effects are related to the dose administered medications, there is an increased risk of infection when and duration of treatment, so the goal is to use it at combining prednisone with other medications that the lowest effective dose for the shortest period of time affect your immune system. Additionally, when taking necessary. Some potential side effects include easy prednisone with NSAIDs (such as naproxen or ibuprofen), bruising, osteoporosis (or weakened bones), diabetes, there can be an increased risk of stomach ulcers. Make hypertension, weight gain, cataracts, glaucoma, and sure to review all of your medications with your physician SIDE a bone disorder called avascular necrosis. -

Celestone Syrup Page 1 Nda 14-215/S-009, 015
CELESTONE SYRUP PAGE 1 NDA 14-215/S-009, 015 CELESTONE® betamethasone syrup, USP DESCRIPTION CELESTONE Syrup, for oral administration, contains 0.6 mg betamethasone in each 5 mL. The inactive ingredients for CELESTONE Syrup include: alcohol (less than 1%), citric acid, FD&C Red No. 40, FD&C Yellow No. 6, flavors, propylene glycol, sodium benzoate, sodium chloride, sorbitol, sugar, and water. The formula for betamethasone is C22H29F05 and it has a molecular weight of 392.47. Chemically, it is 9-fluoro-11β,17,21-trihydroxy-16β-methylpregna-1,4-diene-3,20-dione and has the following structure: (Add structure) Betamethasone is a white to practically white, odorless crystalline powder. It melts at about 240°C with some decomposition. Betamethasone is sparingly soluble in acetone, alcohol, dioxane, and methanol; very slightly soluble in chloroform and ether; and is insoluble in water. CLINICAL PHARMACOLOGY Glucocorticoids, naturally occurring and synthetic, are adrenocortical steroids that are readily absorbed from the gastrointestinal tract. Naturally occurring glucocorticoids (hydrocortisone and cortisone), which also have salt- retaining properties, are used as replacement therapy in adrenocortical deficiency states. Their synthetic analogs, such as betamethasone, are primarily used for their anti-inflammatory effects in disorders of many organ systems. A derivative of prednisolone, betamethasone has a 16β methyl group that enhances the anti-inflammatory action of the molecule and reduces the sodium- and water-retaining properties of the fluorine atom bound at carbon 9. INDICATIONS AND USAGE Allergic states: Control of severe or incapacitating allergic conditions intractable to adequate trials of conventional treatment in asthma, atopic dermatitis, contact dermatitis, drug hypersensitivity reactions, perennial or seasonal allergic rhinitis, serum sickness. -
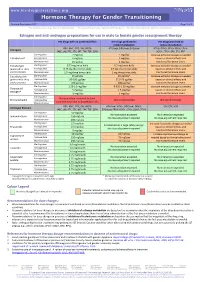
Hormone Therapy for Gender Transitioning Revised September 2017 Page 1 of 2 for Personal Use Only
www.hiv-druginteractions.org Hormone Therapy for Gender Transitioning Revised September 2017 Page 1 of 2 For personal use only. Not for distribution. For personal use only. Not for distribution. For personal use only. Not for distribution. Estrogen and anti-androgen preparations for use in male to female gender reassignment therapy HIV drugs with no predicted effect HIV drugs predicted to HIV drugs predicted to inhibit metabolism induce metabolism RPV, MVC, DTG, RAL, NRTIs ATV/cobi, DRV/cobi, EVG/cobi ATV/r, DRV/r, FPV/r, IDV/r, LPV/r, Estrogens (ABC, ddI, FTC, 3TC, d4T, TAF, TDF, ZDV) SQV/r, TPV/r, EFV, ETV, NVP Starting dose 2 mg/day 1 mg/day Increase estradiol dosage as needed Estradiol oral Average dose 4 mg/day 2 mg/day based on clinical effects and Maximum dose 8 mg/day 4 mg/day monitored hormone levels. Estradiol gel Starting dose 0.75 mg twice daily 0.5 mg twice daily Increase estradiol dosage as needed (preferred for >40 y Average dose 0.75 mg three times daily 0.5 mg three times daily based on clinical effects and and/or smokers) Maximum dose 1.5 mg three times daily 1 mg three times daily monitored hormone levels. Estradiol patch Starting dose 25 µg/day 25 µg/day* Increase estradiol dosage as needed (preferred for >40 y Average dose 50-100 µg/day 37.5-75 µg/day based on clinical effects and and/or smokers) Maximum dose 150 µg/day 100 µg/day monitored hormone levels. Starting dose 1.25-2.5 mg/day 0.625-1.25 mg/day Increase estradiol dosage as needed Conjugated Average dose 5 mg/day 2.5 mg/day based on clinical effects and estrogen† Maximum dose 10 mg/day 5 mg/day monitored hormone levels. -

Steroid Use in Prednisone Allergy Abby Shuck, Pharmd Candidate
Steroid Use in Prednisone Allergy Abby Shuck, PharmD candidate 2015 University of Findlay If a patient has an allergy to prednisone and methylprednisolone, what (if any) other corticosteroid can the patient use to avoid an allergic reaction? Corticosteroids very rarely cause allergic reactions in patients that receive them. Since corticosteroids are typically used to treat severe allergic reactions and anaphylaxis, it seems unlikely that these drugs could actually induce an allergic reaction of their own. However, between 0.5-5% of people have reported any sort of reaction to a corticosteroid that they have received.1 Corticosteroids can cause anything from minor skin irritations to full blown anaphylactic shock. Worsening of allergic symptoms during corticosteroid treatment may not always mean that the patient has failed treatment, although it may appear to be so.2,3 There are essentially four classes of corticosteroids: Class A, hydrocortisone-type, Class B, triamcinolone acetonide type, Class C, betamethasone type, and Class D, hydrocortisone-17-butyrate and clobetasone-17-butyrate type. Major* corticosteroids in Class A include cortisone, hydrocortisone, methylprednisolone, prednisolone, and prednisone. Major* corticosteroids in Class B include budesonide, fluocinolone, and triamcinolone. Major* corticosteroids in Class C include beclomethasone and dexamethasone. Finally, major* corticosteroids in Class D include betamethasone, fluticasone, and mometasone.4,5 Class D was later subdivided into Class D1 and D2 depending on the presence or 5,6 absence of a C16 methyl substitution and/or halogenation on C9 of the steroid B-ring. It is often hard to determine what exactly a patient is allergic to if they experience a reaction to a corticosteroid. -

Indinavir PK Fact Sheet Reviewed March 2016 Page 1 of 2 for Personal Use Only
www.hiv-druginteractions.org Indinavir PK Fact Sheet Reviewed March 2016 Page 1 of 2 For personal use only. Not for distribution. For personal use only. Not for distribution. For personal use only. Not for distribution. Details Generic Name Indinavir Trade Name Crixivan® Class Protease Inhibitor Molecular Weight 711.88 (as sulphate) Structure HO OH N N H H SO N N 2 4 O HN O H3C CH3 H3C Summary of Key Pharmacokinetic Parameters Plasma half life 1.8 ± 0.4 h Cmax 8.97 ± 2.87 µg/ml (800 mg every 8 hours) Cmin 0.146 µg/ml (800 mg every 8 hours) AUC 21.82 ± 8.11 µg/ml.h (800 mg every 8 hours) Bioavailability Approx 65% Absorption Administration of indinavir with a meal high in calories, fat, and protein resulted in a blunted and reduced absorption with an approximate 80 % reduction in AUC and an 86 % reduction in Cmax. Administration with light meals (e.g., dry toast with jam or fruit conserve, apple juice, and coffee with skimmed or fat–free milk and sugar or corn flakes, skimmed or fat–free milk and sugar) resulted in plasma concentrations comparable to the corresponding fasted values. Protein Binding ~60% Volume of Distribution ~1.74 L/kg [1] CSF:Plasma ratio 0.14 [2] Semen:Plasma ratio 1.9 [2] Renal Clearance <20% as unchanged drug Renal Impairment Safety in patients with impaired renal function has not been studied; less than 20% of indinavir is excreted in the urine as unchanged drug or metabolites. Hepatic Impairment Safety and efficacy of indinavir has not been established in patients with significant underlying liver disorders and should be used with caution. -

Drug-Drug Interaction Between Protease Inhibitors and Statins and Proton Pump Inhibitors
Drug-drug interaction between Protease inhibitors and statins and Proton pump inhibitors Item Type text; Electronic Report Authors Orido, Charles; McKinnon, Samantha Publisher The University of Arizona. Rights Copyright © is held by the author. Download date 01/10/2021 01:48:07 Item License http://rightsstatements.org/vocab/InC/1.0/ Link to Item http://hdl.handle.net/10150/636245 Group 47 :Orido/Samantha 1 Drug-drug interaction between Protease inhibitors and statins and Proton pump inhibitors Course Title: PhPr 862 Date: April 3, 2019 Faculty Advisor: Dr. Dan Malone Students: Charles Orido, Samantha McKinnon Pharm.D. Candidates, Class of 2019 Group 47 :Orido/Samantha 2 Objective The purpose of this article is to provide a systematic review of the pharmacokinetic and clinical data on drug-drug interactions between protease inhibitors (PIs) and statins, atazanavir and proton pump inhibitors (PPIs)and their clinical relevance. Methods A literature search was performed using Medline, EMBASE and google scholar, abstracts from 1970 to 2019 of major conferences were searched and FDA drug information package inserts of the manufacturer of every currently available PI was looked at. All data was summarized and verified by at least two investigators. Results A total of 246 references were identified, 8 of which were studies of pharmacokinetic and pharmacodynamics interactions between simvastatin, lovastatin and protease inhibitors and an additional 7 articles that provided pharmacokinetic of proton pump inhibitors and Atazanavir. Conclusions Protease inhibitors increases the AUC and Cmax of simvastatin by approximately 500% and 517% respectively. Therefore, simvastatin and Lovastatin are not recommended for a co-administration with a protease inhibitor. -

YONSA (Abiraterone Acetate) Tablets May Have Different Dosing and Food Effects Than Other Abiraterone Acetate Products
HIGHLIGHTS OF PRESCRIBING INFORMATION hypokalemia before treatment. Monitor blood pressure, serum These highlights do not include all the information needed to use YONSA potassium and symptoms of fluid retention at least monthly. (5.1) safely and effectively. See full prescribing information for YONSA. • Adrenocortical insufficiency: Monitor for symptoms and signs of adrenocortical insufficiency. Increased dosage of corticosteroids YONSA® (abiraterone acetate) tablets, for oral use may be indicated before, during and after stressful situations. (5.2) Initial U.S. Approval: 2011 • Hepatotoxicity: Can be severe and fatal. Monitor liver function and modify, interrupt, or discontinue YONSA dosing as recommended. ----------------------------INDICATIONS AND USAGE-------------------------- YONSA is a CYP17 inhibitor indicated in combination with (5.3) methylprednisolone for the treatment of patients with metastatic castration- resistant prostate cancer (CRPC). (1) ------------------------------ADVERSE REACTIONS------------------------------ The most common adverse reactions (≥ 10%) are fatigue, joint swelling or ----------------------DOSAGE AND ADMINISTRATION---------------------- discomfort, edema, hot flush, diarrhea, vomiting, cough, hypertension, To avoid medication errors and overdose, be aware that YONSA tablets may dyspnea, urinary tract infection and contusion. have different dosing and food effects than other abiraterone acetate products. Recommended dose: YONSA 500 mg (four 125 mg tablets) administered The most common laboratory -

Ep 3027638 B1
(19) TZZ¥Z ¥_T (11) EP 3 027 638 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07J 31/00 (2006.01) C07J 43/00 (2006.01) 27.12.2017 Bulletin 2017/52 (86) International application number: (21) Application number: 13771622.1 PCT/IB2013/056206 (22) Date of filing: 29.07.2013 (87) International publication number: WO 2015/015246 (05.02.2015 Gazette 2015/05) (54) PROCESS FOR THE PREPARATION OF ABIRATERONE AND ABIRATERONE ACETATE VERFAHREN ZUR HERSTELLUNG VON ABIRATERON ODER ABIRATERONACETAT PROCÉDÉ DE PRÉPARATION D’ABIRATÉRONE ET D’ACÉTATE D’ABIRATÉRONE (84) Designated Contracting States: (56) References cited: AL AT BE BG CH CY CZ DE DK EE ES FI FR GB WO-A1-93/20097 WO-A1-2006/021777 GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO WO-A2-2014/207567 PL PT RO RS SE SI SK SM TR • SUN Q ET AL: "Pd(PPh3)4/AgOAc-catalyzed (43) Date of publication of application: coupling of 17-steroidal triflates and alkynes: 08.06.2016 Bulletin 2016/23 Highly efficient synthesis of D-ring unsaturated 17-alkynylsteroids", STEROIDS, ELSEVIER (73) Proprietor: Industriale Chimica S.R.L. SCIENCE PUBLISHERS, NEW YORK, NY, US, vol. 20145 Milano (IT) 75, no. 12, 1 December 2010 (2010-12-01), pages 936-943, XP027221112, ISSN: 0039-128X (72) Inventors: [retrieved on 2010-06-01] • LENNA, Roberto I-20010 S. Giorgio Su Legnano (IT) Remarks: • DI BRISCO, Riccardo Thefile contains technical information submitted after I-28069 Trecate (IT) the application was filed and not included in this specification (74) Representative: Palladino, Saverio Massimo et al Notarbartolo & Gervasi S.p.A.