Fucus Gardneri Using Gamma-Ray Spectroscopy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Peacekeepers Parade
The Bosn’s Call Volume 24, No. 3, Autumn 2017 PEACEKEEPERS PARADE Pictured above is the Colour Party from the Calgary Naval Veterans Association at the Peace- keepers’ Parade held on Sunday, August 13th. Left to Right ~ Cal Annis, Bill Bethell, Art Jor- genson and Master-at-Arms Eric Kahler. Calgary Naval Veterans Association • www.cnva.ca CALGARY NAVAL VETERANS ASSOCIATION Skipper’s www.cnva.ca Autumn 2017 | Corvette Club: 2402 - 2A Street SE, Calgary, AB T2G 4Z2 Log [email protected] ~ 403-261-0530 ~ Fax 403-261-0540 n EXECUTIVE Paris Sahlen, CNVA President F PAST PRESIDENT • Art JORGENSON – 403-281-2468, [email protected] – Charities, Communication. The Bosn’s Call The Bosn’s hope everyone has had a nice warm summer F PRESIDENT • Paris SAHLEN, CD – 403-252-4532, RCNA, HMCS Calgary Liaison, Charities, Stampede. with a little smoke thrown in. Here is an update F EXECUTIVE VICE-PRESIDENT • Ken MADRICK Charities, Honours on the different activities the Club has been do- & Awards, Financial Statements, Galley Vice-Admiral. I ing this year so far. F VICE-PRESIDENT • Tom CONRICK • Sick & Visiting, Colonel Belcher, Charities, Honours & Awards. We still have Remembrance Day, our trip to Banff and our New Year’s Levee. The Club will F TREASURER • Anita VON – 403-240-1967. be closed December 23rd. One other thing—it F SECRETARY • Laura WEAVER. would be nice if we asked our Red Seal chefs if n DIRECTORS there is anything they need help with before leav- F Cal ANNIS – 403-938-0955 • Honours & Awards, Galley Till. ing the Club. -

Corporate Plan Summary, the Quarterly June 22, 2017
2018–2019 — DEFENCE CONSTRUCTION CANADA 2022–2023 CORPORATE PLAN INCLUDING THE OPERATING AND SUMMARY CAPITAL BUDGETS FOR 2018–2019 AN INTRODUCTION TO DEFENCE CONSTRUCTION CANADA Defence Construction Canada (DCC) is a unique maintenance work. Others are more complex with organization in many ways—its business model high security requirements. combines the best characteristics from both the private and public sector. To draw a comparison, DCC has site offices at all active Canadian Armed DCC’s everyday operations are similar to those of Forces (CAF) establishments in Canada and abroad, as a civil engineering consultancy firm. However, as required. Its Head Office is in Ottawa and it maintains a Crown corporation, it is governed by Part X of five regional offices (Atlantic, Quebec, Ontario, Schedule III to the Financial Administration Act. Its Western and National Capital Region), as well as 31 key Client-Partners are the Assistant Deputy Minister site offices located at Canadian Armed Forces (CAF) Infrastructure and Environment (ADM IE) Group at bases, wings, and area support units. The Corporation the Department of National Defence (DND) and the currently employs about 900 people. Communications Security Establishment (CSE). The Corporation also provides services to Shared Services As a Crown corporation, DCC complies with Canada relating to the expansion of the electronic Government of Canada legislation, such as the data centre at CFB Borden. DCC employees do not do Financial Administration Act, Official Languages the hands-on, hammer-and-nails construction work Act, Access to Information Act and Employment at the job site. Instead, as part of an organization that Equity Act, to name a few. -

Trident Fury 2020
• CANADIAN MILITARY’S TRUSTED NEWS SOURCE • NEED Volume 65 Number 48 | December 7, 2020 MORE SPACE? STILL TAKING DONATIONS! newspaper.comnewwsspapaper.com 2020 NDWCC MARPAC NEWSEWWS CFBCFCFB Esquimalt,EsEsqqu Victoria, B.C. NOW OPEN! 4402 Westshore Parkway, Victoria DONATE NOW THRU E-PLEDGE (778) 817-1293 • eliteselfstorage.ca TRIDENT FURY 2020 MS Cole Wood, from the Mine Countermeasures Diving Team, checks equipment in the Containerized Diving System Workshop on board HMCS Whitehorse during Exercise Trident Fury 2020. Beautiful smiles We proudly serve the start here! Island Owned and Operated Canadian Forces Community since 1984. As a military family we understand your cleaning needs during ongoing VIEW OUR FLYER Capital Park service, deployment and relocation. www.mollymaid.ca Dental IN THIS PAPER WEEKLY! 250-590-8566 CapitalParkDental.com (250) 744-3427 check out our newly renovated esquimalt store Français aussi ! Suite 110, 525 Superior St, Victoria [email protected] 2 • LOOKOUT CANADIAN MILITARY’S TRUSTED NEWS SOURCE • CELEBRATING 76 YEARS PROVIDING RCN NEWS December 7, 2020 Force Preservation and Generation IN A PANDEMIC SLt K.B. McHale-Hall Dalhousie University in Halifax, N.S., for medical essential activities to be conducted, and the potential MARPAC Public Affairs school, LCdr Drake jokingly remarks of the common- for members to spend the quarantine period in their alities. “I don’t have any shoes named after me yet, but homes should set household requirements be met. “People first, mission always.” there’s still time.” The strict protocol of a full quarantine eliminates all Amidst a global pandemic, this core philosophy of His career began in the Naval Reserves serving as interactions with others. -

ACTION STATIONS! Volume 37 - Issue 1 Winter 2018
HMCS SACKVILLE - CANADA’S NAVAL MEMORIAL ACTION STATIONS! Volume 37 - Issue 1 Winter 2018 Action Stations Winter 2018 1 Volume 37 - Issue 1 ACTION STATIONS! Winter 2018 Editor and design: Our Cover LCdr ret’d Pat Jessup, RCN Chair - Commemorations, CNMT [email protected] Editorial Committee LS ret’d Steve Rowland, RCN Cdr ret’d Len Canfield, RCN - Public Affairs LCdr ret’d Doug Thomas, RCN - Exec. Director Debbie Findlay - Financial Officer Editorial Associates Major ret’d Peter Holmes, RCAF Tanya Cowbrough Carl Anderson CPO Dean Boettger, RCN webmaster: Steve Rowland Permanently moored in the Thames close to London Bridge, HMS Belfast was commissioned into the Royal Photographers Navy in August 1939. In late 1942 she was assigned for duty in the North Atlantic where she played a key role Lt(N) ret’d Ian Urquhart, RCN in the battle of North Cape, which ended in the sinking Cdr ret’d Bill Gard, RCN of the German battle cruiser Scharnhorst. In June 1944 Doug Struthers HMS Belfast led the naval bombardment off Normandy in Cdr ret’d Heather Armstrong, RCN support of the Allied landings of D-Day. She last fired her guns in anger during the Korean War, when she earned the name “that straight-shooting ship”. HMS Belfast is Garry Weir now part of the Imperial War Museum and along with http://www.forposterityssake.ca/ HMCS Sackville, a member of the Historical Naval Ships Association. HMS Belfast turns 80 in 2018 and is open Roger Litwiller: daily to visitors. http://www.rogerlitwiller.com/ HMS Belfast photograph courtesy of the Imperial -

For an Extra $130 Bucks…
For an Extra $130 Bucks…. Update On Canada’s Military Financial Crisis A VIEW FROM THE BOTTOM UP Report of the Standing Senate Committee on National Security and Defence Committee Members Sen. Colin Kenny – Chair Sen. J. Michael Forrestall – Deputy Chair Sen. Norman K. Atkins Sen. Tommy Banks Sen. Jane Cordy Sen. Joseph A. Day Sen. Michael A. Meighen Sen. David P. Smith Sen. John (Jack) Wiebe Second Session Thirty-Seventh Parliament November 2002 (Ce rapport est disponible en français) Information regarding the committee can be obtained through its web site: http://sen-sec.ca Questions can be directed to: Toll free: 1-800-267-7362 Or via e-mail: The Committee Clerk: [email protected] The Committee Chair: [email protected] Media inquiries can be directed to: [email protected] For an Extra 130 Bucks . Update On Canada’s Military Financial Crisis A VIEW FROM THE BOTTOM UP • Senate Standing Committee on National Security and Defence November, 2002 MEMBERSHIP 37th Parliament – 2nd Session STANDING COMMITTEE ON NATIONAL SECURITY AND DEFENCE The Honourable Colin Kenny, Chair The Honourable J. Michael Forrestall, Deputy Chair And The Honourable Senators: Atkins Banks Cordy Day Meighen Smith* (Not a member of the Committee during the period that the evidence was gathered) Wiebe *Carstairs, P.C. (or Robichaud, P.C.) *Lynch-Staunton (or Kinsella) *Ex Officio Members FOR AN EXTRA $130 BUCKS: UPDATE ON CANADA’S MILITARY FINANCIAL CRISIS A VIEW FROM THE BOTTOM UP TABLE OF CONTENTS INTRODUCTION 7 MONEY ISN’T EVERYTHING, BUT . ............................................ 9 WHEN FRUGAL ISN’T SMART .................................................... -

The Readiness of Canada's Naval Forces Report of the Standing
The Readiness of Canada's Naval Forces Report of the Standing Committee on National Defence Stephen Fuhr Chair June 2017 42nd PARLIAMENT, 1st SESSION Published under the authority of the Speaker of the House of Commons SPEAKER’S PERMISSION Reproduction of the proceedings of the House of Commons and its Committees, in whole or in part and in any medium, is hereby permitted provided that the reproduction is accurate and is not presented as official. This permission does not extend to reproduction, distribution or use for commercial purpose of financial gain. Reproduction or use outside this permission or without authorization may be treated as copyright infringement in accordance with the Copyright Act. Authorization may be obtained on written application to the Office of the Speaker of the House of Commons. Reproduction in accordance with this permission does not constitute publication under the authority of the House of Commons. The absolute privilege that applies to the proceedings of the House of Commons does not extend to these permitted reproductions. Where a reproduction includes briefs to a Standing Committee of the House of Commons, authorization for reproduction may be required from the authors in accordance with the Copyright Act. Nothing in this permission abrogates or derogates from the privileges, powers, immunities and rights of the House of Commons and its Committees. For greater certainty, this permission does not affect the prohibition against impeaching or questioning the proceedings of the House of Commons in courts or otherwise. The House of Commons retains the right and privilege to find users in contempt of Parliament if a reproduction or use is not in accordance with this permission. -
Local History Clipping Files
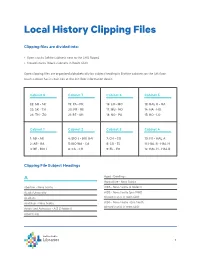
Local History Clipping Files Clipping files are divided into: • Open stacks (white cabinets next to the LHG Room) • Closed stacks (black cabinets in Room 440) Open clipping files are organized alphabetically by subject heading in 8 white cabinets on the 4th floor (each cabinet has its own key at the 4th floor information desk): Cabinet 8 Cabinet 7 Cabinet 6 Cabinet 5 22: SH - SK 19: PA - PR 16: LO - MO 13: HAL R - HA 23: SK - TH 20: PR - RE 17: MU - NO 14: HA - HO 24: TH - ZO 21: RE - SH 18: NO - PA 15: HO - LO Cabinet 1 Cabinet 2 Cabinet 3 Cabinet 4 1: AB - AR 4: BIO J - BIO U-V 7: CH - CO 10: FO - HAL A 2: AR - BA 5: BIO WA - CA 8: CO - EL 11: HAL B - HAL H 3: BE - BIO I 6: CA - CH 9: EL - FO 12: HAL H - HAL R Clipping File Subject Headings A Aged - Dwellings Agriculture - Nova Scotia Abortion - Nova Scotia AIDS - Nova Scotia (2 folders) Acadia University AIDS - Nova Scotia (pre-1990) Acadians (closed stacks in room 440) Acid Rain - Nova Scotia AIDS - Nova Scotia - Eric Smith (closed stacks in room 440) Actors and Actresses - A-Z (3 folders) Advertising 1 Airlines Atlantic Institute of Education Airlines - Eastern Provincial Airways (closed stacks in room 440) (closed stacks in room 440) Atlantic School of Theology Airplane Industry Atlantic Winter Fair (closed stacks in room 440) Airplanes Automobile Industry and Trade - Bricklin Canada Ltd. Airports (closed stacks in room 440) (closed stacks in room 440) Algae (closed stacks in room 440) Automobile Industry and Trade - Canadian Motor Ambulances Industries (closed stacks in room 440) Amusement Parks (closed stacks in room 440) Automobile Industry and Trade - Lada (closed stacks in room 440) Animals Automobile Industry and Trade - Nova Scotia Animals, Treatment of Automobile Industry and Trade - Volvo (Canada) Ltd. -

LAND USE BY-LAW HALIFAX PENINSULA (Edition 223)
LAND USE BY-LAW HALIFAX PENINSULA Halifax Regional Municipality LAND USE BY-LAW HALIFAX PENINSULA (Edition 223) THIS COPY IS A REPRINT OF THE LAND USE BY-LAW WITH AMENDMENTS TO JUNE 17, 2017 LAND USE BY-LAW FOR HALIFAX PENINSULA THIS IS TO CERTIFY that this is a true copy of the Land Use By-law for Halifax Peninsula which was passed by a majority vote of the former City Council at a duly called meeting held on March 30, 1978, and approved by the Minister of Municipal Affairs on August 11, 1978, which includes all amendments thereto which have been adopted by the Halifax Regional Municipality and are in effect as of the 17th day of June, 2017. GIVEN UNDER THE HAND of the Municipal Clerk and under the seal of Halifax Regional Municipality this ____ day of ________________________, 20___. ________________________________ Municipal Clerk The Halifax Regional Municipality, its Officers, and Employees, accept no responsibility for the accuracy of the information contained in this (By-law, Plan, etc.) Please note that HRM Council at its meeting on May 9, 2000, approved a motion to insert the following notation in the Land Use By-law as follows: The provisions of the zones described in this by-law do not apply to property owned or occupied by Her Majesty the Queen in right of the Province of Nova Scotia or Canada in respect of a use of the property made by the Crown. Where a privately owned or occupied property is to be used for a federally regulated activity, the federal jurisdiction may, depending on the particular circumstances, override the requirements of this by-law. -

Buy Wholesale Direct! Over $10.6 Million Inventory Available Same Day
Forget Retail! Buy Wholesale Direct! Over $10.6 million inventory available same day. Family owned for more than 40 years. Value to premium parts available. 902-423-7127 | WWW.CANDRAUTOSUPPLY.CA | 2513 AGRICOLA ST., HALIFAX 144518 Monday, February 19, 2018 Volume 52, Issue 4 www.tridentnewspaper.com Happy Valentine’s Day from HMCS St. John’s MS Jennifer Krick, a sailor deployed on Op REASSURANCE in HMCS St. John’s, sends Valentine’s Day wishes home to her children: Bailey, Brandon, Benjamin and Jessica. CPL TONY CHAND, FIS Bell Let's Talk Day MCDVs depart for Sea trials with Mariners win regional Pg. 2 West Africa Pg. 3 Asterix Pg. 12 hockey Pg. 20 145760 2 TRIDENT NEWS FEBRUARY 19, 2018 Don’t keep quiet about mental health issues, advocate tells CFB Halifax crowd By Ryan Melanson, Trident Staff A Canadian golf pro turned mental health advocate visited CFB Halifax on January 31 to share his story of depression, suicide attempts, and the road to recovery, but he says that finally speaking out loud about his issues, above all else, is what allowed him to start making positive steps and managing his mental health. “The way to heal from this stuff or to start healing from this stuff is just to talk about it. It sounds cliche and all that, and just talking isn’t going to immediately fix your problems, but that’s where it starts,” Andrew Jensen said to the group gathered at Juno Tower for the base event marking Bell Let’s Talk Day. The telecom’s annual social media campaign is aimed at opening discussions and ending stig- mas around these issues, and comes with a hefty donation to mental health initiatives across Canada. -

Map Art, 3 Panel
n T erm in HALIFAX SEAPORT & OCEAN TERMINALS HALTERM COalNs TAINER TERMINAL LIMITED 3 Pie (opreArator of South End Container Terminal) 6 2 Halifax Port Authority d P H e Operator: Halterm Container Terminal Limited Administration Building a h lif S P B ax ier Terminal Size: 74.5 acres / 30.2 hectares ert Halifax Seaport Se A h2 ap -1 0 Farmers' Market k 7 Reefer Outlets: 485 in-ground outlets X 440V L o c OW rt 2 E Pavilion 20 h A R t Throughput Capacity : 750,000 TEU WA r 1 T e 3 3 P ER B 3 S P NSCAD University h ie TR t d r Equipment: • 4 Super Post Panamax (SPPX) EE r e B T Port Campus e h S B e Cranes: 7 high x 22 wide B 3 n . e 8 a r 3 r D Im t 2 C R h h y m 2 Canadian Museum of tr • 3 Panamax Cranes: 5 high x 13 L h t 7 n ig 1 r a A r 3 at Immigration at Pier 21 0 e G M N i h wide I W on O 3 B t M e A c h r R st n Pavilion 22 e t e E in n a B 9 • 3 Ro/Ro ramps T N ex n r o T e 4 3 va M e B 3 4 F A B h Sc R e rm d 3 t o i G • 8,000 ft of on-dock double-stack t l IN P rt i e e r ia m A h n h h n e n L 2 a t ra R l S r 6 C B & O 2 s A e ry rail service (320 TEU) D 3 t M B n h a G S t Ro-Ro e Pa P r Restrictions: No navigational/height restrictions d vilion 23 i e Ramp Be VIA i e o G rth a -R B an e r o try Cr 41 r A m ane Railway C t p e n Gant Pie n 3 ry Station P P e 6 Crane rC Pier B E t 2 r C 2 Ro e h R -Ro G d t d am antry C Be Berth Length Depth (Avg.) Apron Width r r 6 e O p rane rth I a e 2 h A R 42 n S P D o- B h ie R Ro Gan B u t r am try Cran 36 190.5 m / 625 ft 13.9 m / 45 ft Unrestricted A C r A p e RR P e I B -1 N Gantry -

UCSF Radiation Safety Training Manual
` UNIVERSITY OF CALIFORNIA, SAN FRANCISCO RADIATION SAFETY TRAINING MANUAL SEPTEMBER, 1996 This information is being provided in accordance with the following State requirements: CALIFORNIA RADIATION CONTROL REGULATIONS 17CAC30280(a) (1) Each user shall inform individuals working in or frequenting any portion of a controlled area as to the presence of sources of radiation; instruct such individuals in safety problems associated therewith and in precautions or procedures to minimize radiation exposure; and instruct such individuals in the provisions of department regulations and licenses applicable for the protection of personnel. RADIATION SAFETY TRAINING MANUAL TABLE OF CONTENTS SECTION DESCRIPTION PAGE INTRODUCTION 1 CHAPTER 1 PROPERTIES OF IONIZING RADIATION 2 A Structure of the Atom 2 B Atomic Nomenclature 3 C Beta Particles 3 D Radioactive Decay 5 E Gamma and X-rays 5 F Other Modes of Decay 7 G Bremsstrahlung - A Type of X-ray 7 CHAPTER 2 UNITS FOR MEASURING IONIZING RADIATION 8 A Roentgen: The Unit of Exposure 8 B Rad: The Unit of Absorbed Dose 8 C Rem: The Dose Equivalent Unit 9 D Curie: The Unit of Activity 9 CHAPTER 3 MAXIMUM PERMISSIBLE EXPOSURES 11 A Guidelines for Radiation Exposure 11 B Maximum Permissible Dose (MPD) 11 C How Does the MPD Compare with Other Sources of Radiation Exposure? 13 D What is the Risk at the MPD? 14 E Special Safeguards for Pregnant Women 16 CHAPTER 4 BIOLOGICAL EFFECTS OF RADIATION 20 A Somatic and Genetic Effects 20 B Increase in Cancer Incidence 21 C Genetic Damage 21 D Exposure of Unborn Children -

Digital Radiation Meter
Digital Radiation Meter 840024 Instruction Manual Digital Radiation Meter 840024 Copyright ©2011 by Sper Scientific ALL RIGHTS RESERVED Printed in the USA The contents of this manual may not be reproduced or transmitted in any form or by any means electronic, mechanical, or other means that do not yet exist or may be developed, including photocopying, recording, or any information storage and retrieval system without the express permission from Sper Scientific. 7720 E. Redfield Rd. Suite #7, Scottsdale, AZ 85260 Tel: (480) 948-4448 Fax: (480) 967-8736 Web: www.sperscientific.com - 2 - TABLE OF CONTENTS INTRODUCTION. 4 CAUTION . 5 KEYPAD . 6 OPERATING PROCEDURES . 7 METER SETUP . 8 ESTABLISHING BACKGROUND LEVELS . 10 AREA MONITORING . 11 CHECK SURFACE CONTAMINATION . 12 CHECK AN OBJECT . 14 MAINTENANCE AND CALIBRATION . 15 RADIATION AND RADIOACTIVITY . 17 SPECIFICATIONS . 21 WARRANTY . 23 NOTICE . 24 - 3 - INTRODUCTION This manual provides the necessary information for proper use and care of Sper Scientific Model 840024 Radiation Meter. We recommend reading this manual completely prior to using the instrument. It also contains valuable information about the nature of ionizing radiation that should be understood by the user so that accurate measurements can be made. This Radiation Meter features selectable measurement scales, adjustable audible alarm and auto power off. It uses a thin wall glass Geiger-Mueller (GM) tube that will detect Beta and Gamma ionizing radiation and X-rays. The GM tube generates an electrical pulse each time radiation passes through the tube. These pulses are then electronically detected and displayed in either SI units (micro-sieverts per hour) or conventional units (milliroentgens per hour).