The Relation Between Pressure–Volume Curve Traits and Stomatal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

European Hornbeam Carpinus Betulus ‘Fastigiata’
Smart tree selections for communities and landowners European Hornbeam Carpinus betulus ‘Fastigiata’ Height: 35’ Spread: 25’ Site characteristics: Sun to partial shade; prefers moist, well-drained soils Zone: 5a - 7a Wet/dry: Tolerates drought, heavy soil and alkaline soils Native range: Europe, Western Asia Salt: Sensitive pH: 5.0 - 8.2 Shape: Narrow when young, becoming oval Other: Narrow branching angles Additional: Transplant in spring, somewhat slow to establish from bare root Pests: Some occasional dieback Bert Cregg, MSU Bert Cregg, Bert Cregg, MSU Bert Cregg, Bugwood.org Hungary, of West Univ. Norbert Frank, Content development: Dana Ellison, Tree form illustrations: Marlene Cameron. Smart tree selections for communities and landowners Bert Cregg and Robert Schutzki, Michigan State University, Departments of Horticulture and Forestry A smart urban or community landscape has a diverse combination of trees. The devastation caused by exotic pests such as Dutch elm disease, chestnut blight and emerald ash borer has taught us the importance of species diversity in our landscapes. Exotic invasive pests can devastate existing trees because many of these species may not have evolved resistance mechanisms in their native environments. In the recent case of emerald ash borer, white ash and green ash were not resistant to the pest and some communities in Michigan lost up to 20 percent of their tree cover. To promote diverse use of trees by homeowners, landscapers and urban foresters, Michigan State University Extension offers a series of tip sheets for smart urban and community tree selection. In these tip sheets, we suggest trees that should be considered in situations where an ash tree may have been planted in the past. -

The Invasive Alien Leaf Miner Cameraria Ohridella and the Native Tree Acer Pseudoplatanus: a Fatal Attraction?
1 The invasive alien leaf miner Cameraria ohridella and the native tree Acer pseudoplatanus: a fatal attraction? Christelle Per´ e†,´ Sylvie Augustin∗, Ted C. J. Turlings† and Marc Kenis CABI Europe-Switzerland, 2800 Del´emont, Switzerland, ∗INRA, UR 633 Zoologie Foresti`ere, 45000 Orl´eans, France and †Institute of Zoology, University of Neuchˆatel, 2009 Neuchˆatel, Switzerland Abstract 1 The horse-chestnut leaf miner Cameraria ohridella is an invasive moth in Europe and a serious pest of horse-chestnut Aesculus hippocastanum. The moth also occasionally attacks sycamore maple Acer pseudoplatanus, when situated beside infested horse-chestnuts. 2 The main objective of the present study was to provide an overview of the relationship between C. ohridella and A. pseudoplatanus and to determine whether C. ohridella has the potential to shift to this native tree. 3 In the field, females oviposit on different deciduous tree species. Although less frequently attacked than A. hippocastanum, A. pseudoplatanus was clearly preferred for oviposition over 12 other woody species investigated. 4 Surveys in Europe demonstrated that the majority of A. pseudoplatanus trees found beside infested A. hippocastanum had mines of C. ohridella, even though more than 70% of the larvae died within the first two instars. Attack rates and development success greatly varied from site to site. Attack levels on A. pseudoplatanus were not always correlated with those on A. hippocastanum, and mines on A. pseudoplatanus were sometimes observed beside weakly-infested A. hippocastanum. 5 Field observations, experimental exposure of A. pseudoplatanus saplings and rearing trials in a common garden study showed that individual trees may vary in their susceptibility to C. -

Charbrook Nursery
charbrook nursery Plant List Spring-Summer-Autumn 2020 Name Size Wholesale Red Maple 1.5"-2" $200.00 Acer rubrum `Brandywine`, `Frank Jr.`, `Franksred` TM, `Sun Valley` 2"-2.5" $260.00 Single-stem 2.5"-3" $380.00 3"-3.5" $500.00 3.5"-4" $670.00 4"-4.5" $860.00 Sugar Maple 1.5"-2" $250.00 Acer saccharum `Fall Fiesta`, Flashfire 2"-2.5" $300.00 2.5"-3" $380.00 Autumn Blaze Maple 2.5"-3" $360.00 Acer rubrum 'Autumn Blaze' x 'Freemanii' 3"-3.5" $470.00 3.5"-4" $640.00 4"-4.5" $850.00 Shadblow Serviceberry 4'-5' $195.00 Amelanchier x grandiflora 'Autumn Brillance' 5'-6' $240.00 Multi-stem 6'-7' $285.00 7'-8' $420.00 8'-10' $630.00 10'-12' $855.00 Yellow Birch 1"-1.5" $180.00 Betula alleghaniensis 1.5"-2" $250.00 2"-2.5" $350.00 2.5"-3" $460.00 Sweet Birch 1.5"-2" $210.00 Betula lenta 2"-2.5" $300.00 2.5"-3" $400.00 Paper Birch 1.5"-2" $210.00 Betula papyrifera 2"-2.5" $300.00 Betula papyrifera `Varen` 2.5"-3" $400.00 Charbrook Nursery 71 Gates Road Princeton, MA 01541 Page 1 Franz Fontaine Hornbeam 2"-2.5" $320.00 Carpinus betulus `Franz Fontaine` 2.5"-3" $460.00 3"-3.5" $620.00 American Hornbeam 2"-2.5" $300.00 Carpinus caroliniana 2.5"-3" $390.00 3"-3.5" $510.00 Northern Catalpa 2.5"-3" $360.00 Catalpa speciosa 3"-3.5" $470.00 3.5"-4" $690.00 4"-4.5" $900.00 Giant Dogwood 2"-2.5" $280.00 Cornus controversa `June Snow` 2.5"-3" $350.00 Tamarack 10'-12' $400.00 Larix laricina 12'-14' $530.00 (Spring dig only) 14'-16' $710.00 Crab Apple 1.5"-2" $210.00 Malus x `Donald Wyman` , `Schmidtcutleaf` TM, `Snowdrift` 2"-2.5" $260.00 American Hophornbeam -

Hornbeam Carpinus Betulus
34 W I N G E D S E E D S W I N G E D S E E D S 35 Sycamore Acer pseudoplatanus Hornbeam Carpinus betulus Leaves are large and five-lobed, with ornbeams are native trees found largely in south-eastern his introduced species grows in a wide dark green upper range of habitats and soil types. Sycamore sides.The seeds HEngland, with scattered trees in other parts of the country. T come in pairs, that They tolerate a wide range of soils, including sands, gravels and is an excellent coloniser and is often consid- are joined together ered a problem species as, in certain habitats, at an angle. heavy clay, but grow best on damp, fertile soils. Hornbeams including woodland, it can become the domi- produce excellent autumn colours, retaining their leaves throughout much of the winter. nant species. Large tree (5:10:25) Sycamore wood is light in colour, strong and One of the hardest and toughest woods in hard, and is used for kitchen table-tops, floor- Britain, the name hornbeam derives ing, veneers and toys. from the fact that the wood is as This species supports a limited number hard as horn. It was used for of insect species, which includes large cattle yokes, waterwheels and numbers of aphids. In consequence, butchers’ chopping blocks. The migrating warblers can often be found timber also makes excellent fire- feeding in sycamores in the autumn. The wood. tree also supports good lichen growth, Hornbeams are valu- particularly in the west of Britain. able to wildlife, pro- ducing nutlets Seed Guide: Collect the fruits from the which are eaten tree in autumn when they turn by hawfinches brown. -

SP532 Trees to Plant in Containers Or Wells
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Forestry, Trees, and Timber UT Extension Publications 3-1999 SP532 Trees to Plant In Containers or Wells The University of Tennessee Agricultural Extension Service Follow this and additional works at: https://trace.tennessee.edu/utk_agexfores Part of the Plant Sciences Commons Recommended Citation "SP532 Trees to Plant In Containers or Wells," The University of Tennessee Agricultural Extension Service, SP 532-15M-3/99 R12-4910-17-001-00, https://trace.tennessee.edu/utk_agexfores/54 The publications in this collection represent the historical publishing record of the UT Agricultural Experiment Station and do not necessarily reflect current scientific knowledge or ecommendations.r Current information about UT Ag Research can be found at the UT Ag Research website. This Trees for Tennessee Landscapes - Choosing the Right Tree is brought to you for free and open access by the UT Extension Publications at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Forestry, Trees, and Timber by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. Agricultural Extension Service The University of Tennessee SP 532 Trees to Plant In Containers or Wells Donna C. Fare Wayne K. Clatterbuck Research Horticulturist Assistant Professor USDA-ARS Forestry, Wildlife US National Arboretum & Fisheries Donna C. Fare C. Donna Donna C. Fare C. Donna Pleasing example of a group of trees growing in containers. An in-ground tree planter with a magnolia tree. Landscaping in a small area is challenging, but popular. An important aspect of container or well planting is the Planting trees in small areas can limit root and shoot devel- soil or growing medium. -

Symposium on the Gray Squirrel
SYMPOSIUM ON THE GRAY SQUIRREL INTRODUCTION This symposium is an innovation in the regional meetings of professional game and fish personnel. When I was asked to serve as chairman of the Technical Game Sessions of the 13th Annual Conference of the Southeastern Association of Game and Fish Commissioners this seemed to be an excellent opportunity to collect most of the people who have done some research on the gray squirrel to exchange information and ideas and to summarize some of this work for the benefit of game managers and other biologists. Many of these people were not from the southeast and surprisingly not one of the panel mem bers is presenting a general resume of one aspect of squirrel biology with which he is most familiar. The gray squirrel is also important in Great Britain but because it causes extensive damage to forests. Much work has been done over there by Monica Shorten (Mrs. Vizoso) and a symposium on the gray squirrel would not be complete without her presence. A grant from the National Science Foundation through the American Institute of Biological Sciences made it possible to bring Mrs. Vizoso here. It is hoped that this symposium will set a precedent for other symposia at future wildlife conferences. VAGN FLYGER. THE RELATIONSHIPS OF THE GRAY SQUIRREL, SCIURUS CAROLINENSIS, TO ITS NEAREST RELATIVES By DR. ]. C. MOORE INTRODUCTION It seems at least slightly more probable at this point in our knowledge of the living Sciuridae, that the northeastern American gray squirrel's oldest known ancestors came from the Old \Vorld rather than evolved in the New. -

00TS Johnson
Treenet Proceedings of the Inaugural Street Tree Symposium: 7 th and 8 th September 2000 ISBN 0-9775084-0-4 Treenet Inc GREENING THE CITY OF WEST TORRENS Tim Johnson The following notes summarize the presentation given at the TREENET Symposium in September 2000. The presentation summary included: • the current state of established street trees in the City of West Torrens • the City’s historical approach to greening • issues and problems in greening a highly urbanized area with a culturally diverse population • recent greening works • trials of a range of relatively unknown tree species to determine their local suitability for street use The northern boundary of West Torrens follows the River Torrens, the Glenelg tramline forms part of the southern boundary. Soils range from heavy clay to loam & sand. Average annual rainfall recorded at the airport is 450mm • Agonis flexuosa Halifax Street Hilton & Henry Street Plympton are examples of typical streetscapes occurring throughout West Torrens. Many streets have narrow nature strips, severely restricting the range of tree species which can be planted. Many existing street trees were planted in response to publication of the schedules included in Regulation 12 of the Sewerage Act of 1929-1977. The Agonis flexuosa in Goldfinch Avenue at Cowandilla are one example, they were removed during autumn 2000 and replaced with Acer buergerianum • Eucalyptus tessellaris and Eucalyptus cneorifolia Eucalyptus tessellaris in Selby Street Kurralta Park is an example of an inappropriate species for street use. Structural hazards & infrastructure impacts at 20 years of age required that they be removed. Eucalyptus cneorifolia and some other species planted following preparation of the Sewerage Act regulations and schedules remain quite healthy but their contribution to the amenity of streetscapes is frequently questioned. -

Wood Identification and Chemistry' Covers the Physicalproperties and Structural Features of Hardwoods and Softwoods
11 DOCUMENT RESUME ED 031 555 VT 007 853 Woodworking Technology. San Diego State Coll., Calif. Dept. of Industrial Arts. Spons Agency-Office of Education (DHEA Washington, D.C. Pub Date Aug 68 Note-252p.; Materials developed at NDEA Inst. for Advanced Studyin Industrial Arts (San Diego, June 24 -Au9ust 2, 1968). EDRS Price MF -$1.00 He -$13.20 Descriptors-Curriculum Development, *Industrial Arts, Instructional Materials, Learning Activities, Lesson Plans, Lumber Industry, Resource Materials, *Resource Units, Summer Institutes, Teaching Codes, *Units of Study (Sublect Fields), *Woodworking Identifiers-*National Defense Education Act TitleXIInstitute, NDEA TitleXIInstitute, Woodworking Technology SIX teaching units which were developed by the 24 institute participantsare given. "Wood Identification and Chemistry' covers the physicalproperties and structural features of hardwoods and softwoods. "Seasoning" explainsair drying, kiln drying, and seven special lumber seasoning processes. "Researchon Laminates" describes the bending of solid wood and wood laminates, beam lamination, lamination adhesives,. andplasticlaminates."Particleboard:ATeachingUnitexplains particleboard manufacturing and the several classes of particleboard and theiruses. "Lumber Merchandising" outhnes lumber grades andsome wood byproducts. "A Teaching Unitin Physical Testing of Joints, Finishes, Adhesives, and Fasterners" describes tests of four common edge pints, finishes, wood adhesives, and wood screws Each of these units includes a bibhography, glossary, and student exercises (EM) M 55, ...k.",z<ONR; z _: , , . "'zr ss\ ss s:Ts s , s' !, , , , zs "" z' s: - 55 Ts 5. , -5, 5,5 . 5, :5,5, s s``s ss ' ,,, 4 ;.< ,s ssA 11111.116; \ ss s, : , \s, s's \ , , 's's \ sz z, ;.:4 1;y: SS lza'itVs."4,z ...':',\\Z'z.,'I,,\ "t"-...,,, `,. -

The Relationship Among Infection Intensity of Viscum Album with Some Ecological Parameters of Host Trees
Int. J. Environ. Res., 1(2): 143-149, Spring 2007 ISSN: 1735-6865 The Relationship among Infection Intensity of Viscum album with some Ecological Parameters of Host Trees Kartoolinejad, D.1*, Hosseini, S. M.1, Mirnia, S. K.2, Akbarinia, M.1 and Shayanmehr, F.1 1Natural Resources Faculty, Tarbiat Modares University, Tehran, Iran 2Agriculture Faculty, Tarbiat Modares University, Tehran, Iran Received 20 March 2006; Revised 12 Dec 2006; Accepted 10 Jan 2007 ABSTRACT: We investigated the relations among infection intensity of European mistletoe (Viscum album L.) with host tree features in Nour Forest Park, located in Caspian Forests in North of Iran. The number of 30 circular plots with an area of 0.1 ha were sampled in all places have an aggregation of infested trees. Parameters including DBH, height, distance to stand edge, distance to conspecific tree, bark diameter and the number of adult mistletoe per tree for all infected individuals were recorded. Results showed that the mistletoe abundance and infection intensity in Parrotia persica was more than the other host species and also, have positive significant relation with DBH, distance to conspecific and locating in the stand edge, but no significant relation observed about height of host trees. Results of this study suggest that individual differences among host trees (specially DBH) play an important role in explaining local abundance and distribution of mistletoe plants. Key words: Infection intensity, European mistletoe, Host trees, Caspian forests, Conspecific tree *Corresponding author: [email protected] INTRODUCTION Mistletoes are a polyphyletic and diverse real root, parasitize the stems of dicotyledonous group of flowering plants comprising over 1306 trees and shrubs by means of parenchyma organs species from a broad range of habitats across all named haustoria, developing from the radicle of continents except Antarctica. -
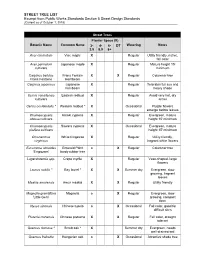
Approved Street Tree and Shrub List, from Public Works Standards
STREET TREE LIST Excerpt from Public Works Standards Section 5 Street Design Standards (Current as of October 1, 2019) Street Trees Planter Space (ft) Botanic Name Common Name 3- 4- 6- DT Watering Notes 3.9 5.9 8+ Acer circinatum Vine maple X Regular Utility friendly, native, fall color Acer palmatum Japanese maple X Regular Mature height 15' cultivars minimum Carpinus betulus Frans Fontain X X Regular Columnar tree Frans Fontaine Hornbeam Carpinus japonicus Japanese X Regular Tolerates full sun and hornbeam heavy shade Cercis canadensis Eastern redbud X Regular Avoid very hot, dry cultivars areas Cercis occidentalis * Western redbud * X Occasional Purple flowers emerge before leaves Chamaecyparis Hinoki cypress X Regular Evergreen, mature obtusa cultivars height 15' minimum Chamaecyparis Sawara cypress X Occasional Evergreen, mature pisifera cultivars height 15' minimum Chionanthus White fringetree X Regular Utility friendly, virginicus fragrant white flowers Eucommia ulmoides Emerald Point o X Regular Columnar tree 'Empozam' hardy rubber tree Lagerstroemia spp. Crepe myrtle X Regular Vase-shaped, large flowers Laurus nobilis * Bay laurel * X X Summer dry Evergreen, slow growing, fragrant leaves Maakia amurensis Amur maakia X X Regular Utility friendly Magnolia grandiflora Magnolia o X Regular Evergreen, slow 'Little Gem' growing, compact form Nyssa sinensis Chinese tupelo o X Occasional Fall color, good for difficult sites Pistacia chinensis Chinese pistache X X Regular Fall color, drought tolerant Quercus dumosa * Scrub oak * X Summer -

Acer Pseudoplatanus (Maple-Sycamore) ID #570
Salve Regina University Digital Commons @ Salve Regina BIO 140 Arboretum Project Student Work on Display 4-27-2020 Acer pseudoplatanus (Maple-Sycamore) ID #570 Caitlyn Rubino Follow this and additional works at: https://digitalcommons.salve.edu/bio140_arboretum Part of the Environmental Monitoring Commons Caitlyn Rubino April 23, 2020 BIO-140L-01: Humans and Their Environment (Lab) Salve Regina University Maple-Sycamore Acer pseudoplatanus ID #570 Over the first half of the spring semester, we were told to select a tree anywhere on campus to observe and take photographs of it every so often. Since time for student’s to be on campus was cut short due to the Coronavirus, we were unable to observe our selected tree after March 13th. The tree that I selected is located on the lawn on Watts-Sherman along Victoria Avenue. I chose this tree because the size of the tree and ivy that covers the trunk stood out to me and I passed by it every day as I was walking to or from my dorm in Wallace Hall, which made it easy to observe. Figure 1: a photo of my tree on February Figure 2: a photo of ivy growing around the 5th, 2020. trunk on April 20th, 2020. I learned more about this tree after examining the details from ArborScope by looking up the tree’s ID number, which is 570. The radius of crown is 10 meters, the height is 20 meters, and the DHB (diameter at breast height) is 40 centimeters. The maple- sycamore (Acer pseudoplatanus) on our campus is mature but in poor condition due to the ivy that is growing around the trunk. -

The Influence of American Chestnut
ARTICLE IN PRESS Pedobiologia 50 (2007) 553—562 www.elsevier.de/pedobi The influence of American Chestnut (Castanea dentata) on nitrogen availability, organic matter and chemistry of silty and sandy loam soils Charles C. Rhoadesà US Forest Service, Rocky Mountain Research Station, 240 W. Prospect, Fort Collins, CO 80526, USA Received 28 September 2006; accepted 1 October 2006 KEYWORDS Summary Tree species effects; American chestnut trees once dominated vast areas of deciduous forest in eastern Soil nitrogen cycling; North America, but the exotic chestnut blight almost eliminated the species from Net mineralization; the region. Introduction of blight-resistant American chestnut hybrids will probably Soil texture; start in the next decade after many years of tree breeding. What were the historic Parent material; effects of chestnut on forest soils, and what changes may follow reintroduction of Forest restoration hybrid chestnuts? A site in southern Wisconsin provided an opportunity to examine the effect of chestnut trees on soil properties. At this site, 600 km northwest of chestnut’s historic distribution, naturalized chestnuts have spread throughout an intact mixed-species forest from nine planted trees. The site contains soil developed on a silty loess-mantled ridge that abuts sandier hillslopes, allowing the effects of individual chestnuts to be examined on two soil types. I sampled and analyzed forest floor and mineral soils beneath canopies of individual American chestnuts and the surrounding mixed-species deciduous forest on fine-silt and sandy-loam soil types. On sandy loam soils, total soil carbon (C) and nitrogen (N), inorganic N and net mineralization and nitrification rates were 10–17% higher beneath chestnut canopies compared to soils beneath mixed-species deciduous forest.