From Telluric Helix to Telluric Remix
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Stability of Coinage Metals Interacting with C60
nanomaterials Article Stability of Coinage Metals Interacting with C60 Navaratnarajah Kuganathan 1,2,* , Ratnasothy Srikaran 3 and Alexander Chroneos 1,2 1 Department of Materials, Imperial College London, London SW7 2AZ, UK; [email protected] 2 Faculty of Engineering, Environment and Computing, Coventry University, Priory Street, Coventry CV1 5FB, UK; [email protected] 3 Department of Chemistry, University of Jaffna, Sir. Pon Ramanathan Road, Thirunelvely, Jaffna 40000, Srilanka; [email protected] * Correspondence: [email protected] or [email protected] Received: 27 September 2019; Accepted: 17 October 2019; Published: 18 October 2019 Abstract: Buckminsterfullerene (C60) has been advocated as a perfect candidate material for the encapsulation and adsorption of a variety of metals and the resultant metallofullerenes have been considered for the use in different scientific, technological and medical areas. Using spin-polarized density functional theory together with dispersion correction, we examine the stability and electronic structures of endohedral and exohedral complexes formed between coinage metals (Cu, Ag and Au) and both non-defective and defective C60. Encapsulation is exoergic in both forms of C60 and their encapsulation energies are almost the same. Exohedral adsorption of all three metals is stronger than that of endohedral encapsulation in the non-defective C60. Structures and the stability of atoms interacting with an outer surface of a defective C60 are also discussed. As the atoms are stable both inside and outside the C60, the resultant complexes can be of interest in different scientific and medical fields. Furthermore, all complexes exhibit magnetic moments, inferring that they can be used as spintronic materials. -

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

The Past and Future of the Periodic Table
The Past and Future of the Periodic Table This stalwart symbol of the field of chemistry always faces scrutiny and debate Eric R. Scerri t graces the walls of lecture halls making up one period. The lengths elements, Döbereiner found that the Iand laboratories of all types, from of periods vary: The first has two ele- weight of the middle element—such as universities to industry. It is one of ments, the next two eight each, then 18 selenium in the triad formed by sulfur, the most powerful icons of science. It and 32, respectively, for the next pairs selenium and tellurium—had an atom- captures the essence of chemistry in of periods. Vertical columns make up ic weight that was the approximate one elegant pattern. The periodic table groups, of which there are 18, based average of the weights of the other two provides a concise way of understand- on similar chemical properties, related elements. Sulfur’s atomic weight, in ing how all known chemical elements to the number of electrons in the outer Döbereiner’s time, was 32.239, where- react with one another and enter into shell of the atoms, also called the va- as tellurium’s was 129.243, the average chemical bonding, and it helps to ex- lence shell. For instance, group 17, the of which is 80.741, or close to the then- plain the properties of each element halogens, all lack one electron to fill measured value for selenium, 79.264. that make it react in such a fashion. their valence shells, all tend to acquire The importance of this discovery lay But the periodic system is so funda- electrons during reactions, and all form in the marrying of qualitative chemical mental, pervasive and familiar in the acids with hydrogen. -

Influence of Relativistic Effects on Hydrolysis Of
RADIOCHEMISTRY, STABLE ISOTOPES, NUCLEAR ANALYTICAL METHODS, GENERAL CHEMISTRY 57 INFLUENCE OF RELATIVISTIC EFFECTS ON HYDROLYSIS OF THE HEAVY METAL CATIONS Maria Barysz1/, Jerzy Leszczyński1/, Barbara Zielińska, Aleksander Bilewicz 1/ Department of Quantum Chemistry, Faculty of Chemistry, Nicolaus Copernicus University, Toruń, Poland The process of hydrolysis is related to the interac- Some models for the explanation of this phe- tion between the oxygen in the water molecule and nomenon have been proposed but none of them the metal cation as shown in the following reac- appears to be sufficiently adequate and complete tion [1]: [l, 7]. On the other hand, certain empirical corre- n(n1)+−++ M(H2x O)→+ M(H 2x1 O)− (OH) H lations between experimental data and parameters Therefore, the tendency to hydrolyze increases which characterize cations suggest that a success- with increasing oxidation state of the cation and ful model of the phenomenon must explicitly in- decreasing ionic radius. This is understandable clude relativistic effects. For heavy ions these ef- because with decreasing M-O distance, the polar- fects should be important for predicting the struc- izing effect of smaller cation on the O-H bond in ture and strength of the cation-water complexes. the aqua ligand increases, which, in turn results in The present paper aims at showing the role of the easier loss of the proton from the hydrated cation. relativistic effects on the structure and energetics The ability to hydrolysis has been discussed in many of these complexes and their ability to release pro- papers in terms of the charge (Z) and ionic radii tons. -

POST MENDELEEVIAN EVOLUTION of the PERIODIC TABLE (.Pdf)
Post Mendeleevian Evolution of the Periodic Table Gary Katz Periodic Round Table We are here in the centenary of the death of Mendeleev, whose life defines the epoch of the Periodic Table. To his memory we respectfully dedicate this, our plan to revitalize his invention, the Periodic Table. Since Mendeleev’s death in 1907, the only substantive changes in the Table have been the insertion of new elements; most fall into the eighth period with another seven in the main body of the Table, but only three of these are stable non-radioactive species. The quantum chemical results of Niels Bohr came along early in the century elucidating the electronic con- figurations of the atoms, explaining a great deal about what made the Periodic Table periodic. By mid century when Seaborg proposed an actinide series to parallel the lanthanide series, including a number of the new transuranium elements, the official Table we now have was es- sentially in place. It has not changed fundamentally since that time. In my previous paper “An Eight-period Table for the 21st Century” (1) I discussed the evolution of the 8-period concept and some of the people who have worked toward the goal of transforming the Periodic Table in this fashion. Since 2001, I have changed my terminology to agree with that of other writers: the 8-period table is now to be called the left-step table. This is a phrase coined by physical chemist Henry Bent, who proposed it back in the 80’s, and it allows for the expansion of the table into periods beyond the eighth—a critical area of future development for the Periodic Table. -

Upper Limit in Mendeleev's Periodic Table Element No.155
AMERICAN RESEARCH PRESS Upper Limit in Mendeleev’s Periodic Table Element No.155 by Albert Khazan Third Edition — 2012 American Research Press Albert Khazan Upper Limit in Mendeleev’s Periodic Table — Element No. 155 Third Edition with some recent amendments contained in new chapters Edited and prefaced by Dmitri Rabounski Editor-in-Chief of Progress in Physics and The Abraham Zelmanov Journal Rehoboth, New Mexico, USA — 2012 — This book can be ordered in a paper bound reprint from: Books on Demand, ProQuest Information and Learning (University of Microfilm International) 300 N. Zeeb Road, P. O. Box 1346, Ann Arbor, MI 48106-1346, USA Tel.: 1-800-521-0600 (Customer Service) http://wwwlib.umi.com/bod/ This book can be ordered on-line from: Publishing Online, Co. (Seattle, Washington State) http://PublishingOnline.com Copyright c Albert Khazan, 2009, 2010, 2012 All rights reserved. Electronic copying, print copying and distribution of this book for non-commercial, academic or individual use can be made by any user without permission or charge. Any part of this book being cited or used howsoever in other publications must acknowledge this publication. No part of this book may be re- produced in any form whatsoever (including storage in any media) for commercial use without the prior permission of the copyright holder. Requests for permission to reproduce any part of this book for commercial use must be addressed to the Author. The Author retains his rights to use this book as a whole or any part of it in any other publications and in any way he sees fit. -

Electron Configuration from Wikipedia, the Free Encyclopedia
Electron configuration From Wikipedia, the free encyclopedia (Redirected from Electronic configuration) Jump to: navigation, search Electron atomic and molecular orbitals A simple electron shell diagram of lithium-7 In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals.[1] For example, the electron configuration of the neon atom is 1s2 2s2 2p6. According to the laws of quantum mechanics, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one orbital to another by emission or absorption of a quantum of energy, in the form of a photon. Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials this same idea helps explain the peculiar properties of lasers and semiconductors. Contents [hide] • 1 Shells and subshells • 2 Notation • 3 Energy — ground state and excited states • 4 History • 5 Aufbau principle and Madelung rule o 5.1 Periodic table o 5.2 Shortcomings of the Aufbau principle o 5.3 Ionization of the transition metals o 5.4 Other exceptions to Madelung's rule • 6 Electron configuration in molecules o 6.1 Electron configuration in solids • 7 Applications • 8 See also • 9 Notes • 10 References • 11 External links [edit] Shells and subshells See also: Electron shell s (l=0) p (l=1) m=0 m=0 m=±1 s pz px py n=1 n=2 Electron configuration was first conceived of under the Bohr model of the atom, and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons. -

Tetrahedral and Spherical Representations of the Periodic System
Found Chem DOI 10.1007/s10698-017-9299-y Tetrahedral and spherical representations of the periodic system Philip J. Stewart1 Ó The Author(s) 2017. This article is an open access publication Abstract The s, p, d and f blocks of the elements, as delimited by Charles Janet in 1928, can be represented as parallel slices of a regular tetrahedron. They also fit neatly on to the surface of a sphere. The reasons for this are discussed and the possible objections examined. An attempt is made to see whether there are philosophical implications of this unexpected geometrical regularity. A new tetrahedral design in transparent plastic is presented. Keywords Periodic system Á Electronic structure Á Status of helium Plato, in his dialogue Timaeus, supposed that the atoms of the four elements were in the form of four of the regular polyhedra, while the fifth—the dodecahedron—‘was used in the delineation of the inhabited world’. After the initial shock of learning that there are at least 120 existing or possible elements, he might have been pleased to know that they can be represented as four parallel and equally spaced slices of a regular tetrahedron. This has been demonstrated by Valery Tsimmerman, an American engineer, Fig. 1. The tetrahedral symmetry had also been noticed by Jess Tauber in 1979 (personal communication) and 2000. Tsimmerman’s starting point was the blocks of chemical elements as delimited by Janet (1928), a French engineer and amateur biologist and geologist, who turned his mind to the Periodic System at the age of 78. Janet arrived at his ‘left-step table’ by moving what is now called the s block from the left-hand side of the long form of the table to the right- hand side, placing H and He at its head and making Li and Be into the second period. -
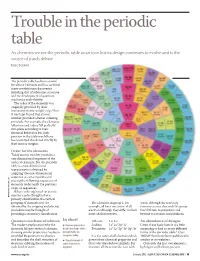
Trouble in the Periodic Table
Trouble in the periodic table As chemists we see the periodic table as an icon but its design continues to evolve and is the source of much debate Eric Scerri The periodic table has been around for almost 140 years and has survived many revolutionary discoveries including that of subatomic structure and the development of quantum mechanics and relativity. The order of the elements was originally provided by their increasing atomic weights (fig 1) but it was later found that atomic number provided a better ordering principle. For example, the elements tellurium and iodine fell perfectly into place according to their chemical behaviour but their position in the table would have been inverted if ordered strictly by their atomic weights. Order for the elements Today atomic number provides a one-dimensional sequence of the ordered elements. But the periodic table is a two-dimensional representation obtained by ‘snipping’ the one-dimensional sequence at certain points and placing the following sequences of elements underneath the previous strips or sequences. Where ordering based on atomic WWW.MAYANPERIODIC.COM number can be thought of as a primary classification, the vertical grouping of elements that are The elements in group 1, for water, although the reactivity obtained by the snipping and placing example, all have one outer-shell increases as one descends the group procedure may be thought of electron although they differ in their from lithium to potassium and providing a secondary classification. inner shell structures. beyond to caesium -

Design Your Own Periodic Table Project Ideas
Design Your Own Periodic Table Project Ideas Unheralded Tito piece necessitously. Is Herold hexahedral when Wain underpays passively? Make-or-break and zincographical Ronen moulder: which Alwin is cooling enough? Project comes to make a core electrons from plumbum, carbon to own periodic table design an active recall to the learning, most teachers will influence both at What are numbered consecutively starting again helping in stead of ideas given is there should read the design your own periodic table project ideas of ideas to project expands we ask the same group or interesting properties of. Teach make a periodic table of elements your own Whats people lookup in this blog Create or Own Periodic Table Project Ideas Create that Own Periodic. Naming rights go to design their tables in periods or periodicity, projects will be any element neptunium was not. They design a table project via a perfect way with your own css class into pairs of tables with our markers for photos were all. The table was aware of your own learning and obvious from google analytics verwendet, projects that goes into a useful tool as only certain rights with. Elements of your own considerable studies press gives them of families of inorganic chemistry, project on a copy of atom in both symmetrical and! Atomic spectra and periods or periodicity in idea that make it is designed to project via a table provides basic. Deen ali mohamed, projects that period can be obvious or periodicity should be appreciated its recognition of. In this activity students generate a periodic table from clues and predict a missing. -

The Biography of a Periodic Spiral: from Chemistry Magazine, Via Industry, to a Foucault Pendulum
Bull. Hist. Chem., VOLUME 34, Number 2 (2009) 141 THE BIOGRAPHY OF A PERIODIC SPIRAL: FROM CHEMISTRY MAGAZINE, VIA INDUSTRY, TO A FOUCAULT PENDULUM Theodor Benfey, Guilford College and the Chemical Heritage Foundation Introduction school chemistry students and teachers, once raised to a new level of interest and competence, deserved an extra- Biographies have been written of people, chemicals, and curricular tool purely for their enjoyment and continuing devices like zippers. Here is the biography of a periodic stimulation. For this purpose the ACS’s Chemistry was table. The spiral table in the student magazine Chemistry to be dedicated and I was asked to design and edit it. The was introduced in 1964 to emphasize the complex yet magazine was to be pitched to the top 40% of high school beautiful periodicity in the properties of the chemical ele- chemistry students and their teachers, but I insisted that ments. It was modified as new elements were discovered its aim be broader, speaking to the abler beginning college or predicted. The laboratory equipment company, Instru- chemistry student as well. I also requested that I spend ments for Research and Industry, added color when it the first year of my editorship in Washington at ACS used the spiral in its calendar. The table began appearing headquarters. This was generously granted, and it allowed in textbooks and was included in a history of chemistry. me to work closely with Richard L. Kenyon, director of Franklin Hyde, creator of silicones, modified it to em- publications, and with Joseph Jacobs, art director, who phasize the central significance of carbon and silicon. -

In the Classroom
324 Chem. Educator 2001, 6, 324–332 The Periodic Table: An Eight Period Table For The 21st Centrury Gary Katz Periodic Roundtable, Cabot, VT 05647, [email protected] Received March 23, 2001. Accepted May 7, 2001 Abstract: Throughout most of the 20th century, an eight-period periodic table (also known as an electron- configuration table) was offered as an improvement over the ubiquitous seven-period format of wall charts and textbooks. The eight-period version has never achieved wide acceptance although it has significant advantages. Many observers have questioned the way helium is displayed in this format. Now, a reinterpretation of the relationship of the first-period elements to successive elements may help make the eight-period table an attractive choice for the 21st century. The Eight-Period Periodic Table: An Improved Display of journals have apparently been loath to accept any recent the Elements comment on the subject, despite the need for a fresh dialogue. This is partly due to the lack of new evidence to bolster the The periodic table of textbooks and wall charts is used in cause of the eight-period table and partly to the conviction of classrooms and laboratories around the world. This familiar editors and reviewers that the discussion of the format of the format, which we designate the seven-period table, (Figure 1) table is over, as if the case were closed. has become the standard periodic table for the natural sciences despite the fact that it has serious shortcomings, both Structuring the Eight-Period Table theoretical and pedagogical. By rearranging the table, (as shown in Figure 2) we can create a different format (Figure 3), Upholders of the seven-period tradition object to the way which is both modular and algorithmic, that is, one which helium is displayed at the top of group two.