01 Excipients Prelims 1..9
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Calcium Stearate Processing
National Organic Standards Board Technical Advisory Panel (TAP) Review Compiled by University of California Sustainable Agriculture Research and Education Program (UC SAREP) for the USDA National Organic Program Calcium Stearate Processing Executive Summary1 A petition is under consideration with respect to NOP regulations subpart G §205.605, governing the use of substances in processed products: Petitioned: Inclusion of calcium stearate on National List of nonagricultural substances allowed in or on processed products labeled as “organic” or “made with organic (specified ingredients or food group(s)).” Calcium stearate is a compound of calcium with a mixture of solid organic acids obtained from edible sources. It is generally used as a solid-phase lubricant that reduces friction between particles of the substance to which it is added. The Petitioner’s intended use is “as a flow agent (anti-dusting agent)” to be used in dry flour based ingredients sold to bakeries (NOSB Petition). The NOP has no prior listing or ruling on the substance. All three reviewers agreed that the substance should be considered synthetic. The reviewers were divided over the use of calcium stearate in food labeled as “organic.” Two of the reviewers felt it should not be allowed in these foods, while one reviewer felt it should be accepted. One reviewer who voted to restrict its use indicated that more information was needed on the nature of the substance and its potential applications, and the other reviewer felt that inclusion of a “synthetic” substance in organics runs contrary to consumer’s expectations regarding organic products. All three reviewers agreed that the substance should be allowed in products labeled as “made with organic…” ingredients. -

Lubricants in Pharmaceutical Solid Dosage Forms
Lubricants 2014, 2, 21-43; doi:10.3390/lubricants2010021 OPEN ACCESS lubricants ISSN 2075-4442 www.mdpi.com/journal/lubricants Review Lubricants in Pharmaceutical Solid Dosage Forms Jinjiang Li * and Yongmei Wu Drug Product Science & Technology, Bristol-Myers Squibb Corporation, 1 Squibb Dr., New Brunswick, NJ 08903, USA; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-732-227-6584; Fax: +1-732-227-3784. Received: 18 December 2013; in revised form: 21 January 2014 / Accepted: 24 January 2014 / Published: 25 February 2014 Abstract: Lubrication plays a key role in successful manufacturing of pharmaceutical solid dosage forms; lubricants are essential ingredients in robust formulations to achieve this. Although many failures in pharmaceutical manufacturing operations are caused by issues related to lubrication, in general, lubricants do not gain adequate attention in the development of pharmaceutical formulations. In this paper, the fundamental background on lubrication is introduced, in which the relationships between lubrication and friction/adhesion forces are discussed. Then, the application of lubrication in the development of pharmaceutical products and manufacturing processes is discussed with an emphasis on magnesium stearate. In particular, the effect of its hydration state (anhydrate, monohydrate, dihydrate, and trihydrate) and its powder characteristics on lubrication efficiency, as well as product and process performance is summarized. In addition, the impact of lubrication on the dynamics of compaction/compression processes and on the mechanical properties of compacts/tablets is presented. Furthermore, the online monitoring of magnesium stearate in a blending process is briefly mentioned. Finally, the chemical compatibility of active pharmaceutical ingredient (API) with magnesium stearate and its reactive impurities is reviewed with examples from the literature illustrating the various reaction mechanisms involved. -

Interagency Committee on Chemical Management
DECEMBER 14, 2018 INTERAGENCY COMMITTEE ON CHEMICAL MANAGEMENT EXECUTIVE ORDER NO. 13-17 REPORT TO THE GOVERNOR WALKE, PETER Table of Contents Executive Summary ...................................................................................................................... 2 I. Introduction .......................................................................................................................... 3 II. Recommended Statutory Amendments or Regulatory Changes to Existing Recordkeeping and Reporting Requirements that are Required to Facilitate Assessment of Risks to Human Health and the Environment Posed by Chemical Use in the State ............................................................................................................................ 5 III. Summary of Chemical Use in the State Based on Reported Chemical Inventories....... 8 IV. Summary of Identified Risks to Human Health and the Environment from Reported Chemical Inventories ........................................................................................................... 9 V. Summary of any change under Federal Statute or Rule affecting the Regulation of Chemicals in the State ....................................................................................................... 12 VI. Recommended Legislative or Regulatory Action to Reduce Risks to Human Health and the Environment from Regulated and Unregulated Chemicals of Emerging Concern .............................................................................................................................. -

Films Built by Depositing Successive Monomolecular Layers on a Solid Surface by KATHARINEB
June, i935 ~EPOSITIONOF SUCCESSIVEMONOMOLECULAR LAYERS 1007 2. In addition to showing the above change, sulfuric acid or stannic chloride is not due to the xanthone and lluorenone possess new bands in ether oxygen. sulfuric acid sohtions which are believed to be 4. The color of ketone chlorides and sulfuric associated with the stabilization of the quinonoid acid or stannic chloride is of a different nature structure for these compounds. Solutions of from that of ketones and resembles that of tri- stannic chloride produce the same changes on the arylcarbinols and salts of these carbinols. It is absorption spectrum of xanthone that sulfuric postulated that in such solutions the ketone acid does. chlorides exist in a quinonoid modification. 3. The production of color with xanthone and ANN ARBOR,MICH. RECEIVEDMARCH 28, 1935 [CONTRIBUTION FROM THE RESEARCHLABORATORY, GENERALELECTRIC COMPANY ] Films Built by Depositing Successive Monomolecular Layers on a Solid Surface BY KATHARINEB. BLODGETT’ A previous paper2 has described briefly a 5.0, approximately, the Ca ions in the water com- method of depositing successive monomolecular bine with the carboxyl. group of the adsorbed layers of “stearic acid” on glass. Subsequent molecules, converting the film to calcium stear- experiment^,^ which will be discussed in this ate. The film may be neutral soap or an acid paper, have shown that the substance deposited soap, depending on the pH of the water. The on the glass was calcium stearate and not stearic term “acid soap” is used here in the sense in which acid. The layers were deposited one at a time; it is used by McBain6 and other writers to refer thus a film could be built having a thickness of 1, to compounds of the fatty acid and the neutral 2, 3 or more layers of molecules. -
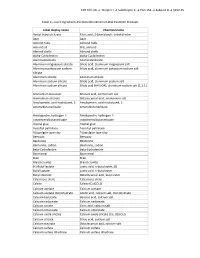
CFR Title 40 → Chapter I → Subchapter E → Part 152 → Subpart B → §152.25
CFR Title 40 → Chapter I → Subchapter E → Part 152 → Subpart B → §152.25 Table 2—Inert Ingredients Permitted in Minimum Risk Pesticide Products Label display name Chemical name Acetyl tributyl citrate Citric acid, 2-(acetyloxy)-, tributyl ester Agar Agar Almond hulls Almond hulls Almond oil Oils, almond Almond shells Almond shells alpha-Cyclodextrin alpha-Cyclodextrin Aluminatesilicate Aluminatesilicate Aluminum magnesium silicate Silicic acid, aluminum magnesium salt Aluminum potassium sodium Silicic acid, aluminum potassium sodium salt silicate Aluminum silicate Aluminum silicate Aluminum sodium silicate Silicic acid, aluminum sodium salt Aluminum sodium silicate Silicic acid (H4 SiO4), aluminum sodium salt (1:1:1) Ammonium benzoate Benzoic acid, ammonium salt Ammonium stearate Octadecanoic acid, ammonium salt Amylopectin, acid-hydrolyzed, 1- Amylopectin, acid-hydrolyzed, 1- octenylbutanedioate octenylbutanedioate Amylopectin, hydrogen 1- Amylopectin, hydrogen 1- octadecenylbutanedioate octadecenylbutanedioate Animal glue Animal glue Ascorbyl palmitate Ascorbyl palmitate Attapulgite-type clay Attapulgite-type clay Beeswax Beeswax Bentonite Bentonite Bentonite, sodian Bentonite, sodian beta-Cyclodextrin beta-Cyclodextrin Bone meal Bone meal Bran Bran Bread crumbs Bread crumbs (+)-Butyl lactate Lactic acid, n-butyl ester, (S) Butyl lactate Lactic acid, n-butyl ester Butyl stearate Octadecanoic acid, butyl ester Calcareous shale Calcareous shale Calcite Calcite (Ca(CO3)) Calcium acetate Calcium acetate Calcium acetate monohydrate Acetic -

Calcium Stearate (Calcii Stearas)
The International Pharmacopoeia - Sixth Edition, 2016 Calcium stearate (Calcii stearas) Calcium stearate (Calcii stearas) C H CaO 36 70 4 Chemical name. Calcium stearate; calcium octadecanoate; CAS Reg. No. 1592-23-0. Description. A white to yellowish white, fine, bulky powder; odour, slight, characteristic. Solubility. Practically insoluble in water, ethanol (~750 g/l) TS, acetone R, and ether R. Category. Tablet and capsule lubricant. Storage. Calcium stearate should be kept in a well-closed container. Additional information. The degree of lubrication depends on the particular form and size of the material. Requirements Definition. Calcium stearate consists of calcium salts mainly of stearic acid and palmitic acid in variable proportions. Calcium stearate contains not less than 9.0% and not more than the equivalent of 10.5% of CaO, calculated with reference to the dried substance. Identity tests A. Heat 1 g with a mixture of 25 mL of water and 5 mL of hydrochloric acid (~420 g/l) TS; fatty acids are liberated and float as an oil on the surface of the liquid. The aqueous layer yields the reactions described under 2.1 General identification tests as characteristic of calcium. B. Mix 25 g with 200 mL of hot water, add 60 mL of sulfuric acid (~100 g/l) TS, and heat the mixture until the separated fatty acids layer is clear. Wash it with boiling water until free from sulfates, transfer it to a beaker, and warm on a water-bath until the water separates and the fatty acids are clear. Allow to cool, pour off the water layer, melt the fatty acids, and filter into a dry beaker. -

EPA Screening-Level Hazard Characterization
U.S. Environmental Protection Agency September, 2011 Hazard Characterization Document SCREENING-LEVEL HAZARD CHARACTERIZATION Lubricating Grease Thickeners Category (See Appendix for list of chemicals) The High Production Volume (HPV) Challenge Program1 was conceived as a voluntary initiative aimed at developing and making publicly available screening-level health and environmental effects information on chemicals manufactured in or imported into the United States in quantities greater than one million pounds per year. In the Challenge Program, producers and importers of HPV chemicals voluntarily sponsored chemicals; sponsorship entailed the identification and initial assessment of the adequacy of existing toxicity data/information, conducting new testing if adequate data did not exist, and making both new and existing data and information available to the public. Each complete data submission contains data on 18 internationally agreed to ―SIDS‖ (Screening Information Data Set1,2) endpoints that are screening-level indicators of potential hazards (toxicity) for humans or the environment. The Environmental Protection Agency’s Office of Pollution Prevention and Toxics (OPPT) is evaluating the data submitted in the HPV Challenge Program on approximately 1400 sponsored chemicals by developing hazard characterizations (HCs). These HCs consist of an evaluation of the quality and completeness of the data set provided in the Challenge Program submissions. They are not intended to be definitive statements regarding the possibility of unreasonable risk of injury to health or the environment. The evaluation is performed according to established EPA guidance2,3 and is based primarily on hazard data provided by sponsors; however, in preparing the hazard characterization, EPA considered its own comments and public comments on the original submission as well as the sponsor’s responses to comments and revisions made to the submission. -

Best Practices for Effective Tablet Lubrication Tony Carpanzano, B.S., R
Best Practices for Effective Tablet Lubrication Tony Carpanzano, B.S., R. Ph. Director R&D, JRS Pharma, LP Agenda 1 Introduction 2 Manufacturing Challenges 3 Lubrication 101 4 Choosing a Lubricant 5 Summary Production Concerns Achieving efficient lubrication for a trouble-free tablet manufacturing process is a search for the middle-ground between picking and sticking, and over-lubrication. Finding that balance can be difficult. Manufacturing Challenges Manufacturing Concerns Less Lubricant Level More Lubricant Effect Picking Defect-free Okay looking Soft tablets Sticking tablets of tablets with with high Capping specified poor friability Laminating hardness disintegration & dissolution Manufacturing Concerns Less Lubricant Level More Lubricant Effect Picking Defect-free Okay looking Soft tablets Sticking tablets of tablets with with high Capping specified poor friability Laminating hardness disintegration & dissolution Unacceptable Acceptable Unacceptable Quality Concerns Manufacturing Concerns Less Lubricant Level More Lubricant Effect Picking Defect-free Okay looking Soft tablets Sticking tablets of tablets with with high Capping specified poor friability Laminating hardness disintegration & dissolution Unacceptable Acceptable Unacceptable Quality Concerns Lubrication Level and Blending Time Continuum Lubricant Level Lubricant Lubricant Blending Time Lubrication Level and Blending Time Continuum Lubricant Level Lubricant Lubricant Blending Time Tablet Lubrication Challenge Issues with API Issues with Lubricant • API may be high dose -

Handbook of Pharmaceutical Excipients, 7Th Ed. Sample Chapter
Mannitol 1 Nonproprietary Names the appearance of the lyophilized plug in a vial.(16–18) A pyrogen- BP: Mannitol free form is available specifically for this use. Mannitol is used in food applications as a bulking agent. JP: D-Mannitol PhEur: Mannitol USP–NF: Mannitol 8 Description Mannitol is D-mannitol. It is a hexahydric alcohol related to 2 Synonyms mannose and is isomeric with sorbitol. Compressol; Cordycepic acid; C*PharmMannidex; E421; Mannitol occurs as a white, odorless, crystalline powder, or free- Emprove; Ludiflash; manita; manitol; manna sugar; mannit; D- flowing granules. It has a sweet taste, approximately as sweet as mannite; mannite; mannitolum; Mannogem; Pearlitol. glucose and half as sweet as sucrose, and imparts a cooling sensation in the mouth. Microscopically, it appears as orthorhom- 3 Chemical Name and CAS Registry Number bic needles when crystallized from alcohol. Mannitol shows polymorphism.(19) D-Mannitol [69-65-8] 4 Empirical Formula and Molecular Weight 9 Pharmacopeial Specifications C6H14O6 182.17 See Table I. See also Section 18. 5 Structural Formula 10 Typical Properties Compressibility see Figure 1. SEM 1: Excipient: mannitol; manufacturer: Merck; magnification: 50Â; M voltage: 3.5 kV. 6 Functional Category Lyophilization aidplasticizing agent; sweetening agent; tablet and capsule diluent; tonicity agent. 7 Applications in Pharmaceutical Formulation or Technology Mannitol is widely used in pharmaceutical formulations and food products. In pharmaceutical preparations it is primarily used as a diluent (10–90% w/w) in tablet formulations, where it is of particular value since it is not hygroscopic and may thus be used with moisture-sensitive active ingredients.(1,2) SEM 2: Excipient: mannitol; manufacturer: Merck; magnification: 500Â; Mannitol may be used in direct-compression tablet applica- voltage: 3.5 kV. -
(12) United States Patent (10) Patent No.: US 9,340,532 B2 Kirkpatrick Et Al
USOO9340532B2 (12) United States Patent (10) Patent No.: US 9,340,532 B2 Kirkpatrick et al. (45) Date of Patent: May 17, 2016 (54) METHODS AND COMPOSITIONS FOR 2007/0213378 A1 9, 2007 Thomas et al. INHIBITING CNKSR1 2007/0293516 A1 12/2007 Knight et al. 2011/0144.066 A1 6/2011 Mahadevan et al. 2012fO189670 A1 7/2012 Kirkpatricket al. (71) Applicant: PHUSIS THERAPEUTICS, INC., San 2013, O184317 A1 7/2013 Mahadevan et al. Diego, CA (US) 2015, 0126563 A1 5, 2015 Mahadevan et al. (72) Inventors: D. Lynn Kirkpatrick, San Diego, CA FOREIGN PATENT DOCUMENTS (US); Martin Indarte, San Diego, CA (US); Nathan T. Ihle, San Diego, CA CA 77.5563 A 1, 1968 DE 2556O11 A1 6, 1977 (US) DE 3443225 A1 6, 1985 EP 2428504 A1 3, 2012 (73) Assignee: Phusis Therapeutics, Inc., San Diego, EP 2477625 T 2012 CA (US) GB 828.963. A 2, 1960 WO WO90, 15600 A2 12, 1990 (*) Notice: Subject to any disclaimer, the term of this WO WOOOf 18376 A1 4/2000 WO WOO2,083.064 A2 10, 2002 patent is extended or adjusted under 35 WO WOO3,O76436 A 9, 2003 U.S.C. 154(b) by 0 days. WO WOO3,O84473 A2 10, 2003 WO WO 2005/090461 A2 9, 2004 (21) Appl. No.: 14/649,349 WO WO 2005/OOO862. A 1, 2005 WO WO 2005.005421 A1 1, 2005 (22) PCT Filed: Dec. 16, 2013 WO WO 2005/097758 A 10/2005 WO WO 2006/046914 A1 5, 2006 WO WO 2007/039.173 A1 4/2007 (86). -
Safety Assessment of Fatty Acids & Soaps As Used in Cosmetics
Safety Assessment of Fatty Acids & Soaps as Used in Cosmetics Status: Scientific Literature Review for Public Comment Release Date: October 2, 2018 Panel Meeting Date: December 3-4, 2018 All interested persons are provided 60 days from the above date to comment on this safety assessment and to identify additional published data that should be included or provide unpublished data which can be made public and included. Information may be submitted without identifying the source or the trade name of the cosmetic product containing the ingredient. All unpublished data submitted to CIR will be discussed in open meetings, will be available at the CIR office for review by any interested party and may be cited in a peer-reviewed scientific journal. Please submit data, comments, or requests to the CIR Executive Director, Dr. Bart Heldreth. The 2018 Cosmetic Ingredient Review Expert Panel members are: Chair, Wilma F. Bergfeld, M.D., F.A.C.P.; Donald V. Belsito, M.D.; Ronald A. Hill, Ph.D.; Curtis D. Klaassen, Ph.D.; Daniel C. Liebler, Ph.D.; James G. Marks, Jr., M.D.; Ronald C. Shank, Ph.D.; Thomas J. Slaga, Ph.D.; and Paul W. Snyder, D.V.M., Ph.D. The CIR Executive Director is Bart Heldreth, Ph.D. This safety assessment was prepared by Christina L. Burnett, Senior Scientific Analyst/Writer. © Cosmetic Ingredient Review 1620 L St NW, Suite 1200 Washington, DC 20036-4702 ph 202.331.0651 fax 202.331.0088 [email protected] INTRODUCTION Most of the fatty acids and soaps (i.e. fatty acid salts) detailed in this safety assessment are reported to function as anticaking agents, emulsion stabilizers, viscosity increasing agents, opacifying agents, and surfactants, according to the web- based International Cosmetic Ingredient Dictionary and Handbook (wINCI; Dictionary; see Table 1).1 Additional functions included hair and skin conditioning agents, binders, slip modifier, antioxidants, fragrance ingredients, colorants, skin protectants, cosmetic biocide, and film formers. -

Calcium Stearate
Calcium stearate sc-268668 Material Safety Data Sheet Hazard Alert Code Key: EXTREME HIGH MODERATE LOW Section 1 - CHEMICAL PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME Calcium stearate STATEMENT OF HAZARDOUS NATURE CONSIDERED A HAZARDOUS SUBSTANCE ACCORDING TO OSHA 29 CFR 1910.1200. NFPA FLAMMABILITY1 HEALTH2 HAZARD INSTABILITY0 SUPPLIER Santa Cruz Biotechnology, Inc. 2145 Delaware Avenue Santa Cruz, California 95060 800.457.3801 or 831.457.3800 EMERGENCY ChemWatch Within the US & Canada: 877-715-9305 Outside the US & Canada: +800 2436 2255 (1-800-CHEMCALL) or call +613 9573 3112 SYNONYMS C36-H70-CaO4, C36-H70-O4.Ca, Ca(C18-H35-02)2, "stearic acid, calcium salt", "calcium distearate", "Ferro Calcium Stearate 60G", "octadecanoic acid, calcium salt", Aquacal, Calstar, Flexichem, "Flexichem CS", "G 339 S", "Nopcote C 104", "Stavinor 30", "Synpro stearate", "Witco G 339S", "metal soap" Section 2 - HAZARDS IDENTIFICATION CHEMWATCH HAZARD RATINGS Min Max Flammability: 1 Toxicity: 2 Body Contact: 2 Min/Nil=0 Low=1 Reactivity: 1 Moderate=2 High=3 Chronic: 2 Extreme=4 CANADIAN WHMIS SYMBOLS 1 of 8 EMERGENCY OVERVIEW RISK Irritating to eyes and respiratory system. POTENTIAL HEALTH EFFECTS ACUTE HEALTH EFFECTS SWALLOWED ■ Accidental ingestion of the material may be damaging to the health of the individual. ■ Ingestion of anionic surfactants may produce diarrhea, bloated stomach,and occasional vomiting. EYE ■ This material can cause eye irritation and damage in some persons. ■ Direct eye contact with some anionic surfactants in high concentration can cause severe damage to the cornea. Low concentrations can cause discomfort, excess blood flow, and corneal clouding and swelling. SKIN ■ Skin contact is not thought to have harmful health effects, however the material may still produce health damage following entry through wounds, lesions or abrasions.