Prevention of Murine Atherosclerosis with Bempedoic Acid
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prevention of Coronary Heart Disease
PREVENTION OF CORONARY PATIENT WITH CHD HEART DISEASE • Obese male patient with previous angina, cardiac catheterization with placement of two stents and now stable. • Notable clinical characteristics: Thomas F . Wh ayne, J r, MD , PhD, FACC Triglycerides 354 mg/dl. Professor of Medicine (Cardiology) HDL 28 mg/dl in men. Gill Heart Institute BP 140/90 mm/Hg. University of Kentucky Fasting glucose 120 mg/dl. November, 2009 • Specific management? Characteristics of Plaques Prone to HIGH SENSENSITIVITY Rupture From Inflammation and LDL CREACTIVE PROTEIN (hs-CRP) Fibrous cap Media • A MARKER OF INFLAMMATION: CRP AND Lumen Lipid hsCRP ARE SAME PROTEIN Core • MAY BE ANOTHER RISK FACTOR AND PLAY A ROLE IN PLAQUE FORMATION “Vulnerable” plaque • MAY PREDICT HIGH RISK ACUTE CORONARY SYNDROME • MAY INDICATE PATIENTS MOST LIKELY TO Lumen RESPOND TO STATINS T lymphocyte Lipid • STATINS SHOWN TO REDUCE CRP Core Macrophage foam cell (tissue factor) “Activated” Intimal SMC (HLA-DR+) – EZETIMIBE ACCENTUATES THIS EFFECT* “Stable” plaque Normal medial SMC • NEED STATIN DOSE RESPONSE CURVE Libby P. Circulation. 1999; 91:284491:2844--2850.2850. *Sager PT, et al. Am J Cardiol. 2003;92:1414-1418. Resultados Estudio VYTAL sobre PCR: Ezetimiba/Simvastatina vs. Atorvastatina en pts con DM II e Hipercolesterolemia (n=1229) CURRENT CLINICALLY USEFUL MARKERS OF INFLAMMATION Atorvastatina E/Simvas Atorvas E/S 10 mg 20 mg 10/20 40 mg 10/40 0 • LIPOPROTEIN-ASSOCIATED Ezetimiba/ --55 Simvastatina PHOSPHOLIPASE A2*. Atorvastatina ® asales --1010 a Sensibilidad – PLAC TEST. t b --1515 --13.713.7 de Al --13.813.8 • HIGH SENSITIVITY C-REACTIVE --2020 # PCR PROTEIN (hsCRP) . -

Cholesteryl Ester-Transfer Protein Inhibitors Stimulate Aldosterone Biosynthesis in Adipocytes Through Nox-Dependent Processes S
Supplemental material to this article can be found at: http://jpet.aspetjournals.org/content/suppl/2015/01/23/jpet.114.221002.DC1 1521-0103/353/1/27–34$25.00 http://dx.doi.org/10.1124/jpet.114.221002 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS J Pharmacol Exp Ther 353:27–34, April 2015 Copyright ª 2015 by The American Society for Pharmacology and Experimental Therapeutics Cholesteryl Ester-Transfer Protein Inhibitors Stimulate Aldosterone Biosynthesis in Adipocytes through Nox-Dependent Processes s Francisco J. Rios, Karla B. Neves, Aurelie Nguyen Dinh Cat, Sarah Even, Roberto Palacios, Augusto C. Montezano, and Rhian M. Touyz Institute of Cardiovascular and Medical Sciences, British Heart Foundation Glasgow Cardiovascular Research Centre, University of Glasgow, Glasgow, Scotland, United Kingdom (F.J.R., A.N.D.C., S.E., A.C.M., R.M.T.); Faculty of Pharmaceutical Sciences of Ribeirao Preto, University of Sao Paulo, Ribeirao Preto, Brazil (K.B.N.); and Departamento de Bioquímica, Fisiología y Genética Molecular Facultad de CC. de la Salud, Universidad Rey Juan Carlos, Madrid, Spain (R.P.) Downloaded from Received October 29, 2014; accepted January 22, 2015 ABSTRACT Hyperaldosteronism and hypertension were unexpected side whereby CETP inhibitors mediate effects, cells were pretreated effects observed in trials of torcetrapib, a cholesteryl ester-transfer with inhibitors of Nox1/Nox4 [GKT137831; 2-(2-chlorophenyl)-4-[3- jpet.aspetjournals.org protein (CETP) inhibitor that increases high-density lipoprotein. (dimethylamino)phenyl]-5-methyl-1H-pyrazolo[4,3-c]pyridine- Given that CETP inhibitors are lipid soluble, accumulate in adipose 3,6(2H,5H)-dione], Nox1 (ML171 [2-acetylphenothiazine]), mitochondria tissue, and have binding sites for proteins involved in adipogenesis, (rotenone), and STAT3 (S3I-201 [2-hydroxy-4-(((4-methylphenyl) and that adipocytes are a source of aldosterone, we questioned sulfonyloxy)acetyl)amino)-benzoic acid]). -

A Novel Therapeutic Drug for the Treatment of Familial Hypercholesterolemia, Hyperlipidaemia, and Hypercholesterolemia
International Journal of Pharmacy and Pharmaceutical Sciences ISSN- 0975-1491 Vol 8, Issue 3, 2016 Review Article MIPOMERSEN: A NOVEL THERAPEUTIC DRUG FOR THE TREATMENT OF FAMILIAL HYPERCHOLESTEROLEMIA, HYPERLIPIDAEMIA, AND HYPERCHOLESTEROLEMIA AJAY KUMAR1, ARUN ACHARYA2, DINOBANDHU NANDI3, NEHA SHARMA4, EKTA CHITKARA1* 1Department of Paramedical Sciences, Lovely Professional University, Phagwara-144411, Punjab (India) Email: [email protected] Received: 25 Dec 2015 Revised and Accepted: 13 Jan 2016 ABSTRACT Familial Hypercholesterolemia (FH) is one of the most common autosomal dominant disorders which exist in either heterozygous form or a homozygous form. These two forms are prevalent in 1 in 500 and 1 in a million population respectively. FH results in premature atherosclerosis; as early as childhood in case of homozygous (HoFH) form and in adults in case of heterozygous (HeFH) form. In case of HoFH both the alleles for LDL- receptor are defective, whereas the mutation in the single allele is the cause for HeFH. Both the forms of the disease are associated with high levels of LDL-C and lipoprotein (a) in plasma, with high morbidity and mortality rate caused by cardiovascular disease. In several past years, different lipid-lowering drugs like Statins (HMG-coenzyme-A reductase inhibitor), MTTP inhibitor, CETP inhibitors, PCSK9 inhibitor, thyroid mimetics, niacin, bile acid sequestrants and lipid apheresis were administered to patients with FH, to achieve the goal of reducing plasma LDL-C and lipoprotein (a). However, such drugs proved inefficient to achieve the goals because of several reasons. Mipomersen is a 20 nucleotide antisense oligonucleotide; a novel lipid-lowering therapeutic drug currently enrolled in the treatment of patients with HoFH, HeFH and other forms of hypercholesterolemia. -

Next-Generation Sequencing Transforms Today's Biology
Evolving new therapies for the prevention of atherosclerosis: a glimpse of the near future G.K. Hovingh MD PhD ([email protected]) Department of Vascular Medicine AMC Amsterdam The Netherlands Today BMJ 2013;347:f544 www.chinadaily.com.cn/life/2009- 04/21/content_7698500.htm What we know - CVD major burden - LDL-C causally related with CVD - LDL-C goals: the lower the better - Statins : corner stone in therapy How well do we do? Reduction in MACE statin vs placebo (%) 0 -30 Potential for further risk reduction -100 Where do we go? Reduction in MACE statin vs placebo (%) 0 Potential for further -30 risk reduction -50 = further LDL-C lowering? and or Additional Rx? -100 A glance at the future… Atherosclerosis • LDL • HDL • TG • Lp(a) • Inflammation Lipid Modifying Drugs • Cholesterol absorption inhibitors • Squalene synthase inhibitors (SSI) • Microsomal triglyceride transfer protein (MTP) inhibitors • Acyl coenzyme A acyltransferase (ACAT) inhibitors • Diacylglycerol acyltransferase (DGAT) inhibitors • Thyroxin receptor agonists • ApoB mRNA antisense drugs • PCSK9 antibodies • ApoA1-based strategies (iv) • Cholesterol ester transfer protein (CETP) inhibitors ApoA-1 based therapy ApoA1 Mimetics, such as APL-180 Novartis Full-length ApoA1, such as ApoA1 Cerenis Therapeutics Pre-Beta HDL, as generated by delipidation, HDL Therapeutics Inc. Reconstituted HDL, CSL Ltd. ApoA1 Milano MDCO216, The Medicines Company Trimeric ApoA1, Borean Pharma and now Roche RVX-208, as developed by Resverlogix Fx-5A, as developed by Kinemed Inc. HDL -
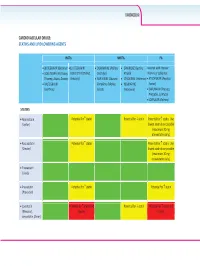
Statins and Lipid Lowering Agents
CARDIOVASCULAR Z/Ks^h>ZZh'^͗ ^dd/E^E>/W/>KtZ/E''Ed^ /E^d/Ɛ EEZd/Ɛ W/Ɛ x/d'Zs/Z ;ŝŬƚĂƌǀLJͿ x>s/d'Zs/Zͬ x KZs/Z/E ;WŝĨĞůƚƌŽ͕ x &s/ZE ;^ƵƐƚŝǀĂ͕ ŽŽƐƚĞĚǁŝƚŚƌŝƚŽŶĂǀŝƌ xK>hd'Zs/Z ;dŝǀŝĐĂLJ͕ K//^dd ;^ƚƌŝďŝůĚ͕ ĞůƐƚƌŝŐŽͿ ƚƌŝƉůĂͿ ;EŽƌǀŝƌͿŽƌĐŽďŝĐŝƐƚĂƚ dƌŝƵŵĞƋ͕:ƵůƵĐĂ͕ŽǀĂƚŽͿ 'ĞŶǀŽLJĂͿ x Z/>W/s/Z/E ;ĚƵƌĂŶƚ͕ x dZs/Z/E ;/ŶƚĞůĞŶĐĞͿ xdEs/Z ;ZĞLJĂƚĂnj͕ xZ>d'Zs/Z ŽŵƉůĞƌĂ͕KĚĞĨƐĞLJ͕ x Es/ZW/E ǀŽƚĂnjͿ ;/ƐĞŶƚƌĞƐƐͿ :ƵůƵĐĂͿ ;sŝƌĂŵƵŶĞͿ xZhEs/Z ;WƌĞnjŝƐƚĂ͕ WƌĞnjĐŽďŝdž͕^LJŵƚƵnjĂͿ x>KW/Es/Z ;<ĂůĞƚƌĂͿ ^dd/E^ xƚŽƌǀĂƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ pƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ͘hƐĞ ;>ŝƉŝƚŽƌͿ ůŽǁĞƐƚƐƚĂƚŝŶĚŽƐĞƉŽƐƐŝďůĞ ;ŵĂdžŝŵƵŵϮϬŵŐ ĂƚŽƌǀĂƐƚĂƚŝŶĚĂŝůLJͿ͘ xZŽƐƵǀĂƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ͘hƐĞ ;ƌĞƐƚŽƌͿ ůŽǁĞƐƚƐƚĂƚŝŶĚŽƐĞƉŽƐƐŝďůĞ ;ŵĂdžŝŵƵŵϭϬŵŐ ƌŽƐƵǀĂƐƚĂƚŝŶĚĂŝůLJͿ͘ xWŝƚĂǀĂƐƚĂƚŝŶ ;>ŝǀĂůŽͿ xWƌĂǀĂƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ ;WƌĂǀĂĐŚŽůͿ x>ŽǀĂƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶĂŶĚ WŽƚĞŶƚŝĂůĨŽƌ pƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶĂŶĚ ;DĞǀĂĐŽƌͿ͕ ƚŽdžŝĐŝƚLJ ƚŽdžŝĐŝƚLJ ƐŝŵǀĂƐƚĂƚŝŶ ;ŽĐŽƌͿ CARDIOVASCULAR INSTIs NNRTIs PIs xBICTEGRAVIR (Biktarvy) xELVITEGRAVIR/ x DORAVIRINE (Pifeltro, x EFAVIRENZ (Sustiva, Boosted with ritonavir xDOLUTEGRAVIR (Tivicay, COBICISTAT (Stribild, Delstrigo) Atripla) (Norvir) or cobicistat Triumeq, Juluca) Genvoya) x RILPIVIRINE (Edurant, x ETRAVIRINE (Intelence) xATAZANAVIR (Reyataz, xRALTEGRAVIR Complera, Odefsey, x NEVIRAPINE Evotaz) (Isentress) Juluca) (Viramune) xDARUNAVIR (Prezista, Prezcobix, Symtuza) xLOPINAVIR (Kaletra) FIBRATES xFenofibrate, bezafibrate, gemfibrozil CHOLESTEROL ABSORPTION INHIBITOR xEzetimibe -

1 CETP Inhibition Improves HDL Function but Leads to Fatty Liver and Insulin Resistance in CETP-Expressing Transgenic Mice on A
Page 1 of 55 Diabetes CETP inhibition improves HDL function but leads to fatty liver and insulin resistance in CETP-expressing transgenic mice on a high-fat diet Lin Zhu1,2, Thao Luu2, Christopher H. Emfinger1,2, Bryan A Parks5, Jeanne Shi2,7, Elijah Trefts3, Fenghua Zeng4, Zsuzsanna Kuklenyik5, Raymond C. Harris4, David H. Wasserman3, Sergio Fazio6 and John M. Stafford1,2,3,* 1VA Tennessee Valley Healthcare System, 2Division of Diabetes, Endocrinology, & Metabolism, 3Department of Molecular Physiology and Biophysics, 4Devision of Nephrology and Hypertension, Vanderbilt University School of Medicine. 5Division of Laboratory Sciences, Centers for Disease Control and Prevention. 6The Center for Preventive Cardiology at the Knight Cardiovascular Institute, Oregon Health & Science University. 7Trinity College of Art and Science, Duke University. * Address correspondence and request for reprints to: John. M. Stafford, 7445D Medical Research Building IV, Nashville, TN 37232-0475, phone (615) 936-6113, fax (615) 936- 1667 Email: [email protected] Running Title: CETP inhibition and insulin resistance Word Count: 5439 Figures: 7 Tables: 1 1 Diabetes Publish Ahead of Print, published online September 13, 2018 Diabetes Page 2 of 55 Abstract In clinical trials inhibition of cholesteryl ester transfer protein (CETP) raises HDL cholesterol levels but doesn’t robustly improve cardiovascular outcomes. About 2/3 of trial participants were obese. Lower plasma CETP activity is associated with increased cardiovascular risk in human studies, and protective aspects of CETP have been observed in mice fed a high-fat diet (HFD) with regard to metabolic outcomes. To define if CETP inhibition has different effects depending on the presence of obesity, we performed short- term anacetrapib treatment in chow- and HFD-fed CETP-transgenic mice. -

PHARMACEUTICAL APPENDIX to the TARIFF SCHEDULE 2 Table 1
Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2020) Revision 19 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -

Clinofibrate Improved Canine Lipid Metabolism in Some but Not All Breeds
NOTE Internal Medicine Clinofibrate improved canine lipid metabolism in some but not all breeds Yohtaro SATO1), Nobuaki ARAI2), Hidemi YASUDA3) and Yasushi MIZOGUCHI4)* 1)Graduate School of Agriculture, Meiji University, 1-1-1 Higashimita, Tama-ku, Kawasaki, Kanagawa 214-8571, Japan 2)Spectrum Lab Japan, 1-5-22-201 Midorigaoka, Meguro-ku, Tokyo 152-0034, Japan 3)Yasuda Veterinary Clinic, 1-5-22 Midorigaoka, Meguro-ku, Tokyo 152-0034, Japan 4)School of Agriculture, Meiji University, 1-1-1 Higashimita, Tama-ku, Kawasaki, Kanagawa 214-8571, Japan ABSTRACT. The objectives of this study were to assess if Clinofibrate (CF) treatment improved J. Vet. Med. Sci. lipid metabolism in dogs, and to clarify whether its efficacy is influenced by canine characteristics. 80(6): 945–949, 2018 We collected medical records of 306 dogs and performed epidemiological analyses. Lipid values of all lipoproteins were significantly decreased by CF medication, especially VLDL triglyceride doi: 10.1292/jvms.17-0703 (TG) concentration (mean reduction rate=54.82%). However, 17.65% of dogs showed drug refractoriness in relation to TG level, and Toy Poodles had a lower CF response than other breeds (OR=5.36, 95% CI=2.07–13.90). Therefore, our study suggests that genetic factors may have an Received: 22 December 2017 effect on CF response, so genetic studies on lipid metabolism-related genes might be conducted Accepted: 9 March 2018 to identify variations in CF efficacy. Published online in J-STAGE: KEY WORDS: clinofibrate, descriptive epidemiology, drug response, dyslipidemia, Toy Poodle 26 March 2018 High serum cholesterol (Cho) and triglyceride (TG) concentrations in dogs are caused by various factors such as lack of exercise, high fat diets, obesity, neutralization, age, diseases and breed [6, 21, 24]. -

Dysfunctional High-Density Lipoproteins in Type 2 Diabetes Mellitus: Molecular Mechanisms and Therapeutic Implications
Journal of Clinical Medicine Review Dysfunctional High-Density Lipoproteins in Type 2 Diabetes Mellitus: Molecular Mechanisms and Therapeutic Implications Isabella Bonilha 1 , Francesca Zimetti 2,* , Ilaria Zanotti 2 , Bianca Papotti 2 and Andrei C. Sposito 1,* 1 Atherosclerosis and Vascular Biology Laboratory (AtheroLab), Cardiology Department, State University of Campinas (Unicamp), Campinas 13084-971, Brazil; [email protected] 2 Department of Food and Drug, University of Parma, 43124 Parma, Italy; [email protected] (I.Z.); [email protected] (B.P.) * Correspondence: [email protected] (F.Z.); [email protected] (A.C.S.); Tel.: +39-05-2190-6172 (F.Z.); +55-19-3521-7098 (A.C.S.); Fax: +55-1-9328-9410 (A.C.S.) Abstract: High density lipoproteins (HDLs) are commonly known for their anti-atherogenic prop- erties that include functions such as the promotion of cholesterol efflux and reverse cholesterol transport, as well as antioxidant and anti-inflammatory activities. However, because of some chronic inflammatory diseases, such as type 2 diabetes mellitus (T2DM), significant changes occur in HDLs in terms of both structure and composition. These alterations lead to the loss of HDLs’ physiological functions, to transformation into dysfunctional lipoproteins, and to increased risk of cardiovascular disease (CVD). In this review, we describe the main HDL structural/functional alterations observed in T2DM and the molecular mechanisms involved in these T2DM-derived modifications. Finally, the main available therapeutic interventions targeting HDL in diabetes are discussed. Citation: Bonilha, I.; Zimetti, F.; Keywords: high density lipoprotein; type 2 diabetes mellitus; HDL function; glycation; oxidation; Zanotti, I.; Papotti, B.; Sposito, A.C. -

Therapy with Cholesteryl Ester Transfer Protein (CETP) Inhibitors And
Therapy with cholesteryl ester transfer protein (CETP) inhibitors and diabetes risk Walter Masson, Martín Lobo, Daniel Siniawski, Melina Huerín, Graciela Molinero, Rene Valero, Juan Nogueira To cite this version: Walter Masson, Martín Lobo, Daniel Siniawski, Melina Huerín, Graciela Molinero, et al.. Therapy with cholesteryl ester transfer protein (CETP) inhibitors and diabetes risk. Journal of Diabetes & Metabolism, OMICS International, 2018, 44 (6), epub ahead of print. 10.1016/j.diabet.2018.02.005. hal-01757621 HAL Id: hal-01757621 https://hal-amu.archives-ouvertes.fr/hal-01757621 Submitted on 10 Apr 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Copyright Cholesteryl ester transfer protein therapy and diabetes risk.A Meta-analysis. Walter Massonab*, Martín Lobob, Daniel Siniawskiab, Melina Huerína, Graciela Molineroa, René Valéroc, Juan P Nogueirabd. aCouncil of Epidemiology and Cardiovascular Prevention, Argentine Society of Cardiology. Azcuenaga 980, C1115AAD Buenos Aires, Argentina bArgentine Society of Lipids. Ambrosio Olmos 820, X5000JGQ Córdoba, Argentina. cAix-Marseille University, UMR 1062 INSERM, 1260 INRA, C2VN, NORT, Marseille, France; Department of Nutrition, Metabolic Diseases, Endocrinology, CHU La Conception, APHM, Marseille, France. dFacultad de Ciencias de la Salud, Universidad Nacional de Formosa. -

Effects of Clofibrate Derivatives on Hyperlipidemia Induced by a Cholesterol-Free, High-Fructose Diet in Rats
Showa Univ. J. Med. Sci. 7(2), 173•`182, December 1995 Original Effects of Clofibrate Derivatives on Hyperlipidemia Induced by a Cholesterol-Free, High-Fructose Diet in Rats Hideyukl KURISHIMA,Sadao NAKAYAMA,Minoru FURUYA and Katsuji OGUCHI Abstract: The effects of the clofibrate derivatives fenofibrate (FF), bezafibrate (BF), and clinofibrate (CF), on hyperlipidemia induced by a cholesterol-free, high-fructose diet (HFD) in rats were investigated. Feeding of HFD for 2 weeks increased the high-density lipoprotein subfraction (HDL1) and decreased the low-density lipoprotein (LDL) fraction. The levels of total cholesterol (TC), free cholesterol, triglyceride (TG), and phospholipid in serum were increased by HFD feeding. Administration of CF inhibited the increase in HDL1 content. All three agents inhibited the decrease in LDL level. Both BF and CF decreased VLDL level. Administration of FF, BF, or CF inhibited the increases of serum lipids, especially that of TC and TG. The inhibitory effects of CF on HFD- induced increases in HDL1, TC, and TG were greater than those of FF and BF. These results demonstrate that FF, BF, and CF improve the intrinsic hyper- lipidemia induced by HFD feeding in rats. Key words: fenofibrate, bezafibrate, clinofibrate, fructose-induced hyperlipide- mia, lipoprotein. Introduction Clofibrate is one of the most effective antihypertriglycedemic agents currently available. However, because of its adverse effects, such as hepatomegaly1, several derivatives, such as clinofibrate (CF) and bezafibrate (BF) have been developed which are more effective and have fewer adverse effects. For example, it has been shown that the hypolipidemic effect of CF is greater than that of clofibrate while its tendency to produce hepatomegaly is less1. -

Pcsk9 Inhibitors Lori Fiallo, Pharmd.,Bcps Aq-Cardiology
7/6/2016 2016 ANNUAL MEETING NEW DRUGS IN CARDIOLOGY PCSK9 INHIBITORS LORI FIALLO, PHARMD.,BCPS AQ-CARDIOLOGY 2016 ANNUAL MEETING OBJECTIVES PCSK9 AND HYPERCHOLESTEROLEMIA • Identify recent FDA approvals in cardiology • PCSK9 • Describe the impact of recent FDA approvals on current practice • Discovery reported in 2003 and 2004 • Design a treatment plan utilizing new medications • PCSK9 missense/LOF mutations- ARIC study • African Americans : 28% less LDL-C, 88% lower risk of developing CVD • Whites with less severe mutation: 15% reduction in LDL-C; 47% reduced risk of CVD • PCSK9 – mediated cholesterol effects revealed • PCSK9 facilitates LDL-R degradation • GOF mutations lead to less LDL-R available to remove LDL-C • LOF mutations lead to increase LDL-R available to remove LDL-C Abifadal M,et al.Nat Genet.2003:34:154-6 Cohen JC, et al.NEJM.2006;354:1264-72. Horton JD,et al.J Lipid Res.2009;50(Suppl)S172-77 2016 ANNUAL MEETING 2016 ANNUAL MEETING PRESENTATION OUTLINE • Hyperlipidemia Medications • PCSK9 inhibitors • Pipeline agents • Heart Failure Medications • Ivaradine (Corlanor) • Sacubitril/valsartan (Entresto) • Antidotes • Idarucizumab (Praxbind) • Andexanet Alfa • Other Pipeline agents 2016 ANNUAL MEETING 2016 ANNUAL MEETING 1 7/6/2016 PCSK9 INHIBITORS ALIROCUMAB (PRALUENT) Name Drug Company Stage of Development • Adverse Reactions • Diarrhea (5%) • Increased serum transaminases (2%) Alirocumab (Praluent) Renergon/Sanofi Approved July 2015 • Hypersensitivity reactions • Influenza (6%) Evolocumab (Repatha) Amgen Approved • Injection