210 PART 329—HABIT-FORMING DRUGS Subpart A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
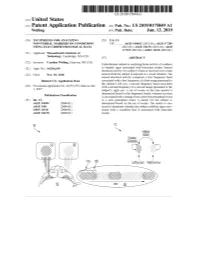
ANNNNNNNNNNNNNNNNNNNN 100A 006 Left Eye Input Right Eye Input
US 20190175049A1 ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2019 /0175049 A1 Welling ( 43 ) Pub . Date : Jun . 13 , 2019 ( 54 ) TECHNIQUES FOR ANALYZING (52 ) U . S . CI. NON -VERBAL MARKERS OF CONDITIONS CPC . .. A61B 5 /04842 (2013 . 01 ) ; A61B 5 / 7289 USING ELECTROPHYSIOLOGICAL DATA (2013 . 01) ; A61B 5 /0478 ( 2013 .01 ) ; A61B 5 /7225 ( 2013. 01 ) ; G06N 20 / 10 (2019 .01 ) (71 ) Applicant: Massachusetts Institute of Technology , Cambridge , MA (US ) ( 57 ) ABSTRACT (72 ) Inventor : Caroline Welling, Hanover, NH (US ) Embodiments related to analyzing brain activity of a subject to identify signs associated with binocular rivalry . Sensed ( 21 ) Appl. No. : 16 / 206, 639 electrical activity of a subject' s brain is received over a time period while the subject is exposed to a visual stimulus. The ( 22 ) Filed : Nov. 30 , 2018 sensed electrical activity comprises a first frequency band Related U . S . Application Data associated with a first frequency of a first image presented to the subject ' s left eye , a second frequency band associated (60 ) Provisional application No .62 / 593 , 535, filed on Dec . with a second frequency of a second image presented to the 1 , 2017 subject ' s right eye . A set of events in the time period is determined based on the frequency bands, wherein an event Publication Classification is associated with a change from a previous perceptual event (51 ) Int. Ci. to a new perceptual event. A metric for the subject is A61B 5 /0484 ( 2006 .01 ) determined based on the set of events . The metric is ana A61B 5 /00 ( 2006 .01 ) lyzed to determine whether the subject exhibits signs asso GO6N 20 / 10 (2006 .01 ) ciated with a condition that is associated with binocular A61B 5 /0478 ( 2006 .01 ) rivalry . -

Sulphonmethane, Sulphonal, Diethylsulpho
is a combination of amylene hydrate and chloral hydrate, superior to the corresponding compounds of other elements. while chloralose, a combination of chloral hydrate and glucose, Experience has shown that, in the main, these claims were un- partakes of the action of morphin and is rather expensive. founded, though many, even now, claim that strontium bro- Chloretone, a more recent product, is not entirely devoid of mid disturbs the stomach less than the corresponding sodium danger and is not always so certain in its action as chloral or potassium salt. Another claim that is frequently made by hydrate, while butyl chloral hydrate, or crotón chloral hydrate, manufacturers of nostrums, like "Peacock's Bromides," is that is one of the older compounds that has been found wanting and they use "chemically pure" salts. Exactly what is meant by is now little used. Of the official compounds of this group we this claim is difficult to say, but the Pharmacopeia gives us a have: number of readily applied tests by which the salts themselves Chloralamid and Paraldehyd. may be tested. The manufacturers of nostrums, on the other Chlobalformamidum.—TJ. S.—Chloralformamid. Chlorala- hand, not infrequently add the very substances that are consid- mid. This has practically the same action as therapeutic ered contaminations. doses of chloral hydrate, the latter being formed in the body by {To be continued.) decomposition of chloralformamid. Average dose: 1 gm. (15 grains). Paraldehtdum.—TJ. S.—Paraldehyd is slower in its action transparent liquid, slower in its action than chloral hydrate, but also safer. It has the disadvantage of a persistently dis- A NEW NEEDLE HOLDER. -

Synthesis, Physicochemical Characterization and Study of the Antimicrobial Activity of Chlorobutanol H
World Academy of Science, Engineering and Technology International Journal of Pharmacological and Pharmaceutical Sciences Vol:12, No:3, 2018 Synthesis, Physicochemical Characterization and Study of the Antimicrobial Activity of Chlorobutanol H. Nadia, G. Bahdja, S. Thili Malha, Y. Zahoua, D. Taoufik, B. Mourad, M. Marzouk, F. Z. Hadjadj Aoul, L. R. Mekacher I. INTRODUCTION Abstract—Introduction and objectives: Chlorobutanol is a raw XCIPIENTS play a vital role in the design, manufacture material, mainly used as an antiseptic and antimicrobial preservative and preservation of drugs. The addition of an in injectable and ophthalmic preparations. The main objective of our E study was the synthesis and evaluation of the antimicrobial activity of antimicrobial agents is of great interest in the case where the chlorobutanol hemihydrates. Material and methods: Chlorobutanol pharmaceutical preparations do not have adequate was synthesized according to the nucleophilic addition reaction of antimicrobial properties, (especially aqueous preparations), to chloroform to acetone, identified by an infrared absorption using prevent proliferation or limit microbial contamination [1], [2]. Spectrum One FTIR spectrometer, melting point, Scanning electron Chlorobutanol is a trihalogen alcohol with bacteriostatic and microscopy and colorimetric reactions. The dosage of Carvedilol fungistatic activity. The main objective of our work was to active substance was carried out by assaying the degradation products of chlorobutanol in a basic solution. The chlorobutanol obtained was obtain chlorobutanol hemihydrate by chemical synthesis, its subjected to bacteriological tests in order to study its antimicrobial purification and characterization as well as the study of its activity. The antibacterial activity was evaluated against strains such antimicrobial activity. as Escherichia coli (ATCC 25 922), Staphylococcus aureus (ATCC 25 923) and Pseudomonas aeroginosa (ATCC = American type II. -

The In¯Uence of Medication on Erectile Function
International Journal of Impotence Research (1997) 9, 17±26 ß 1997 Stockton Press All rights reserved 0955-9930/97 $12.00 The in¯uence of medication on erectile function W Meinhardt1, RF Kropman2, P Vermeij3, AAB Lycklama aÁ Nijeholt4 and J Zwartendijk4 1Department of Urology, Netherlands Cancer Institute/Antoni van Leeuwenhoek Hospital, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands; 2Department of Urology, Leyenburg Hospital, Leyweg 275, 2545 CH The Hague, The Netherlands; 3Pharmacy; and 4Department of Urology, Leiden University Hospital, P.O. Box 9600, 2300 RC Leiden, The Netherlands Keywords: impotence; side-effect; antipsychotic; antihypertensive; physiology; erectile function Introduction stopped their antihypertensive treatment over a ®ve year period, because of side-effects on sexual function.5 In the drug registration procedures sexual Several physiological mechanisms are involved in function is not a major issue. This means that erectile function. A negative in¯uence of prescrip- knowledge of the problem is mainly dependent on tion-drugs on these mechanisms will not always case reports and the lists from side effect registries.6±8 come to the attention of the clinician, whereas a Another way of looking at the problem is drug causing priapism will rarely escape the atten- combining available data on mechanisms of action tion. of drugs with the knowledge of the physiological When erectile function is in¯uenced in a negative mechanisms involved in erectile function. The way compensation may occur. For example, age- advantage of this approach is that remedies may related penile sensory disorders may be compen- evolve from it. sated for by extra stimulation.1 Diminished in¯ux of In this paper we will discuss the subject in the blood will lead to a slower onset of the erection, but following order: may be accepted. -

Determination of Barbiturates and 11-Nor-9-Carboxy-Δ9-THC in Urine
Determination of Barbiturates and 11-Nor-9-carboxy- 9-THC in Urine using Automated Disposable Pipette Extraction (DPX) and LC/MS/MS Fred D. Foster, Oscar G. Cabrices, John R. Stuff, Edward A. Pfannkoch GERSTEL, Inc., 701 Digital Dr. Suite J, Linthicum, MD 21090, USA William E. Brewer Department of Chemistry and Biochemistry, University of South Carolina, 631 Sumter St. Columbia, SC 29208, USA KEYWORDS DPX, LC/MS/MS, Sample Preparation, High Throughput Lab Automation ABSTRACT This work demonstrates the use of disposable pipette extraction (DPX) as a fast and automated sample preparation technique for the determination of barbiturates and 11-nor-9- carboxy- 9-THC (COOH-THC) in urine. Using a GERSTEL MultiPurpose Sampler (MPS) with DPX option coupled to an Agilent 6460 LC-MS/MS instrument, 8 barbiturates and COOH-THC were extracted and their concentrations determined. The resulting average cycle time of 7 min/ sample, including just-in-time sample preparation, enabled high throughput screening. Validation results show that the automated DPX-LC/ AppNote 1/2013 MS/MS screening method provides adequate sensitivity for all analytes and corresponding internal standards that were monitored. Lower limits of quantitation (LLOQ) were found to be 100 ng/mL for the barbiturates and 10 ng/mL for COOH-THC and % CVs were below 10 % in most cases. INTRODUCTION The continuously growing quantity of pain management sample extract for injection [1-2]. The extraction of the drugs used has increased the demand from toxicology Barbiturates and COOH-THC is based on the DPX-RP- laboratories for more reliable solutions to monitor S extraction method described in an earlier Application compliance in connection with substance abuse and/or Note detailing monitoring of 49 Pain Management diversion. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

Smumedical Journal
SMU Medical Journal ISSN : 2349 – 1604 (Volume – 4, No. 1, January 2017) Review Article Indexed in SIS (USA), ASI (Germany), I2OR & i-Scholar (India), SJIF (Morocco) and Cosmos Foundation (Germany) databases. Impact Factor: 3.835 (SJIF) Analytical Aspects with Brief Overview of Depressants Sandeep Kumar1 Nand Gopal Giri2 Ashok Kumar Jaiswal3* Anil Kumar Jaiswal4 1M.Sc. (Forensic Science), LNJN NICFS, New Delhi 110085, 2Assistant Professor, Department of Chemistry, Shivaji College (University of Delhi) Raja Garden, New Delhi 110 027, 3Dept. of Forensic Medicine and toxicology, All India institute of Medical Sciences, New Delhi 110 029.4Assistant Professor, Department of Mathematics, St. Andrew’s PG College, Gorakhpur, UP. *Corresponding author Manuscript received : 30.10.2016 Manuscript accepted: 21.11.2016 Abstract Depressants are drugs that slow down the functions of the central nervous system (CNS). These drugs are used to reduce anxiety and insomnia without drowsiness. The depressants cause relaxed feeling if used in small quantity but cause unconsciousness, vomiting and even death if taken in high quantity. It affects concentration and coordination of a person by slowing down his/ her ability to respond in unexpected situations. These drugs are also attributed for their physiological and psychological effects, eventually in large dose it become lethal. The different 142 SMU Medical Journal, Volume – 4, No. – 1, January, 2017 physical and chemical features of some very often used depressants are discussed in this manuscript. Keyword: Depressant, TLC, UV spectroscopy, HPLC, GLC etc. Introduction The classical depressants are hypnotics (which induce sleep), most antianxiety medicine (diazepam or valium), muscle spasm prevent seizure, but these drugs rapidly develop dependence and tolerance which finally leads to coma and death, so use of these drugs is highly unsafe. -

WO 2012/148799 Al 1 November 2012 (01.11.2012) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2012/148799 Al 1 November 2012 (01.11.2012) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 9/107 (2006.01) A61K 9/00 (2006.01) kind of national protection available): AE, AG, AL, AM, A 61 47/10 (2006.0V) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, (21) International Application Number: DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, PCT/US2012/034361 HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, (22) International Filing Date: KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, 20 April 2012 (20.04.2012) MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SC, SD, (25) Filing Language: English SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, (26) Publication Language: English TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 61/480,259 28 April 201 1 (28.04.201 1) US kind of regional protection available): ARIPO (BW, GH, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, (71) Applicant (for all designated States except US): BOARD UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, OF REGENTS, THE UNIVERSITY OF TEXAS SYS¬ TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, TEM [US/US]; 201 West 7th St., Austin, TX 78701 (US). -

Partial Agreement in the Social and Public Health Field
COUNCIL OF EUROPE COMMITTEE OF MINISTERS (PARTIAL AGREEMENT IN THE SOCIAL AND PUBLIC HEALTH FIELD) RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies, and superseding Resolution AP (82) 2) AND APPENDIX I Alphabetical list of medicines adopted by the Public Health Committee (Partial Agreement) updated to 1 July 1988 APPENDIX II Pharmaco-therapeutic classification of medicines appearing in the alphabetical list in Appendix I updated to 1 July 1988 RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (superseding Resolution AP (82) 2) (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies) The Representatives on the Committee of Ministers of Belgium, France, the Federal Republic of Germany, Italy, Luxembourg, the Netherlands and the United Kingdom of Great Britain and Northern Ireland, these states being parties to the Partial Agreement in the social and public health field, and the Representatives of Austria, Denmark, Ireland, Spain and Switzerland, states which have participated in the public health activities carried out within the above-mentioned Partial Agreement since 1 October 1974, 2 April 1968, 23 September 1969, 21 April 1988 and 5 May 1964, respectively, Considering that the aim of the Council of Europe is to achieve greater unity between its members and that this -

CAS No.) Screening Justification Is Public Comment Now Open? CAS No
EGLE Air Quality Division (AQD) Air Toxics Screening Level Justifications (Numerically by CAS No.) Screening Justification Is Public Comment Now Open? CAS No. Chemical Name Level & Responses Info E-Mail AQD Deadline None ad acid View View Not Open for Public Comment None amyl acetate (mixture) View View Not Open for Public Comment None atlox 848 View View Not Open for Public Comment None biosam tp-1.5 View View Not Open for Public Comment None calcium chloride View View Not Open for Public Comment None epoxy resin solution View View Not Open for Public Comment None heptamethyl-1-vinyl-1,7-dichlorotetrasilazane View View Not Open for Public Comment None n-butylglucamine View View Not Open for Public Comment None n-chloro-2,6-difluorobenzamide View View Not Open for Public Comment None trichloroethylene View View Not Open for Public Comment None triethylammonium suleptanate View View Not Open for Public Comment 50-00-0 formaldehyde View View Not Open for Public Comment 50-03-3 hydrocortisone acetate View View Not Open for Public Comment 50-21-5 lactic acid View View Not Open for Public Comment 50-28-2 estradiol View View Not Open for Public Comment 50-29-3 ddt View View Not Open for Public Comment 50-32-8 benzo(a)pyrene View View Not Open for Public Comment 51-28-5 2,4-dinitrophenol View View Not Open for Public Comment 53-36-1 methyl predisolone acetate View View Not Open for Public Comment 53-70-3 dibenz(a,h)anthracene View View Not Open for Public Comment 56-23-5 carbon tetrachloride View View Not Open for Public Comment 56-49-5 3-methylcholanthrene View View Not Open for Public Comment 56-55-3 benz(a)anthracene View View Not Open for Public Comment 56-81-5 glycerol View View Not Open for Public Comment 57-11-4 stearic acid View View Not Open for Public Comment Revised Monday, September 27, 2021 Page 1 of 51 EGLE Air Quality Division (AQD) Air Toxics Screening Level Justifications (Numerically by CAS No.) Screening Justification Is Public Comment Now Open? CAS No. -

Prohibited Substances List
Prohibited Substances List This is the Equine Prohibited Substances List that was voted in at the FEI General Assembly in November 2009 alongside the new Equine Anti-Doping and Controlled Medication Regulations(EADCMR). Neither the List nor the EADCM Regulations are in current usage. Both come into effect on 1 January 2010. The current list of FEI prohibited substances remains in effect until 31 December 2009 and can be found at Annex II Vet Regs (11th edition) Changes in this List : Shaded row means that either removed or allowed at certain limits only SUBSTANCE ACTIVITY Banned Substances 1 Acebutolol Beta blocker 2 Acefylline Bronchodilator 3 Acemetacin NSAID 4 Acenocoumarol Anticoagulant 5 Acetanilid Analgesic/anti-pyretic 6 Acetohexamide Pancreatic stimulant 7 Acetominophen (Paracetamol) Analgesic/anti-pyretic 8 Acetophenazine Antipsychotic 9 Acetylmorphine Narcotic 10 Adinazolam Anxiolytic 11 Adiphenine Anti-spasmodic 12 Adrafinil Stimulant 13 Adrenaline Stimulant 14 Adrenochrome Haemostatic 15 Alclofenac NSAID 16 Alcuronium Muscle relaxant 17 Aldosterone Hormone 18 Alfentanil Narcotic 19 Allopurinol Xanthine oxidase inhibitor (anti-hyperuricaemia) 20 Almotriptan 5 HT agonist (anti-migraine) 21 Alphadolone acetate Neurosteriod 22 Alphaprodine Opiod analgesic 23 Alpidem Anxiolytic 24 Alprazolam Anxiolytic 25 Alprenolol Beta blocker 26 Althesin IV anaesthetic 27 Althiazide Diuretic 28 Altrenogest (in males and gelidngs) Oestrus suppression 29 Alverine Antispasmodic 30 Amantadine Dopaminergic 31 Ambenonium Cholinesterase inhibition 32 Ambucetamide Antispasmodic 33 Amethocaine Local anaesthetic 34 Amfepramone Stimulant 35 Amfetaminil Stimulant 36 Amidephrine Vasoconstrictor 37 Amiloride Diuretic 1 Prohibited Substances List This is the Equine Prohibited Substances List that was voted in at the FEI General Assembly in November 2009 alongside the new Equine Anti-Doping and Controlled Medication Regulations(EADCMR). -

Intravenous Anesthetics a Drug That Induces Reversible Anesthesia the State of Loss of Sensations, Or Awareness
DR.MED. ABDELKARIM ALOWEIDI AL-ABBADI ASSOCIATE PROF. FACULTY OF MEDICINE THE UNIVERSITY OF JORDAN Goals of General Anesthesia Hypnosis (unconsciousness) Amnesia Analgesia Immobility/decreased muscle tone (relaxation of skeletal muscle) Reduction of certain autonomic reflexes (gag reflex, tachycardia, vasoconstriction) Intravenous Anesthetics A drug that induces reversible anesthesia The state of loss of sensations, or awareness. Either stimulates an inhibitory neuron, or inhibits an excitatory neuron. Organs are divided according to blood perfusion: High- perfusion organs (vessel-rich); brain takes up disproportionately large amount of drug compared to less perfused areas (muscles, fat, and vessel-poor groups). After IV injection, vessel-rich group takes most of the available drug Drugs bound to plasma proteins are unavailable for uptake by an organ After highly perfused organs are saturated during initial distribution, the greater mass of the less perfused organs continue to take up drug from the bloodstream. As plasma concentration falls, some drug leaves the highly perfused organs to maintain equilibrium. This redistribution from the vessel-rich group is responsible for termination of effect of many anesthetic drugs. Compartment Model Inhibitory channels : GABA-A channels ( the main inhibitory receptor). Glycine channels. Excitatory channels : Neuronal nicotic. NMDA. Rapid onset (mainly unionized at physiological pH) High lipid solubility Rapid recovery, no accumulation during prolonged infusion Analgesic