Hexarelin-Tesamorelin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Drug Testing Program
DRUG TESTING PROGRAM Copyright © 2021 CrossFit, LLC. All Rights Reserved. CrossFit is a registered trademark ® of CrossFit, LLC. 2021 DRUG TESTING PROGRAM 2021 DRUG TESTING CONTENTS 1. DRUG-FREE COMPETITION 2. ATHLETE CONSENT 3. DRUG TESTING 4. IN-COMPETITION/OUT-OF-COMPETITION DRUG TESTING 5. REGISTERED ATHLETE TESTING POOL (OUT-OF-COMPETITION DRUG TESTING) 6. REMOVAL FROM TESTING POOL/RETIREMENT 6A. REMOVAL FROM TESTING POOL/WATCH LIST 7. TESTING POOL REQUIREMENTS FOLLOWING A SANCTION 8. DRUG TEST NOTIFICATION AND ADMINISTRATION 9. SPECIMEN ANALYSIS 10. REPORTING RESULTS 11. DRUG TESTING POLICY VIOLATIONS 12. ENFORCEMENT/SANCTIONS 13. APPEALS PROCESS 14. LEADERBOARD DISPLAY 15. EDUCATION 16. DIETARY SUPPLEMENTS 17. TRANSGENDER POLICY 18. THERAPEUTIC USE EXEMPTION APPENDIX A: 2020-2021 CROSSFIT BANNED SUBSTANCE CLASSES APPENDIX B: CROSSFIT URINE TESTING PROCEDURES - (IN-COMPETITION) APPENDIX C: TUE APPLICATION REQUIREMENTS Drug Testing Policy V4 Copyright © 2021 CrossFit, LLC. All Rights Reserved. CrossFit is a registered trademark ® of CrossFit, LLC. [ 2 ] 2021 DRUG TESTING PROGRAM 2021 DRUG TESTING 1. DRUG-FREE COMPETITION As the world’s definitive test of fitness, CrossFit Games competitions stand not only as testaments to the athletes who compete but to the training methodologies they use. In this arena, a true and honest comparison of training practices and athletic capacity is impossible without a level playing field. Therefore, the use of banned performance-enhancing substances is prohibited. Even the legal use of banned substances, such as physician-prescribed hormone replacement therapy or some over-the-counter performance-enhancing supplements, has the potential to compromise the integrity of the competition and must be disallowed. With the health, safety, and welfare of the athletes, and the integrity of our sport as top priorities, CrossFit, LLC has adopted the following Drug Testing Policy to ensure the validity of the results achieved in competition. -

To Download a List of Prescription Drugs Requiring Prior Authorization
Essential Health Benefits Standard Specialty PA and QL List July 2016 The following products require prior authorization. In addition, there may be quantity limits for these drugs, which is notated below. Therapeutic Category Drug Name Quantity Limit Anti-infectives Antiretrovirals, HIV SELZENTRY (maraviroc) None Cardiology Antilipemic JUXTAPID (lomitapide) 1 tab/day PRALUENT (alirocumab) 2 syringes/28 days REPATHA (evolocumab) 3 syringes/28 days Pulmonary Arterial Hypertension ADCIRCA (tadalafil) 2 tabs/day ADEMPAS (riociguat) 3 tabs/day FLOLAN (epoprostenol) None LETAIRIS (ambrisentan) 1 tab/day OPSUMIT (macitentan) 1 tab/day ORENITRAM (treprostinil diolamine) None REMODULIN (treprostinil) None REVATIO (sildenafil) Soln None REVATIO (sildenafil) Tabs 3 tabs/day TRACLEER (bosentan) 2 tabs/day TYVASO (treprostinil) 1 ampule/day UPTRAVI (selexipag) 2 tabs/day UPTRAVI (selexipag) Pack 2 packs/year VELETRI (epoprostenol) None VENTAVIS (iloprost) 9 ampules/day Central Nervous System Anticonvulsants SABRIL (vigabatrin) pack None Depressant XYREM (sodium oxybate) 3 bottles (540 mL)/30 days Neurotoxins BOTOX (onabotulinumtoxinA) None DYSPORT (abobotulinumtoxinA) None MYOBLOC (rimabotulinumtoxinB) None XEOMIN (incobotulinumtoxinA) None Parkinson's APOKYN (apomorphine) 20 cartridges/30 days Sleep Disorder HETLIOZ (tasimelteon) 1 cap/day Dermatology Alkylating Agents VALCHLOR (mechlorethamine) Gel None Electrolyte & Renal Agents Diuretics KEVEYIS (dichlorphenamide) 4 tabs/day Endocrinology & Metabolism Gonadotropins ELIGARD (leuprolide) 22.5 mg -

Classification Decisions Taken by the Harmonized System Committee from the 47Th to 60Th Sessions (2011
CLASSIFICATION DECISIONS TAKEN BY THE HARMONIZED SYSTEM COMMITTEE FROM THE 47TH TO 60TH SESSIONS (2011 - 2018) WORLD CUSTOMS ORGANIZATION Rue du Marché 30 B-1210 Brussels Belgium November 2011 Copyright © 2011 World Customs Organization. All rights reserved. Requests and inquiries concerning translation, reproduction and adaptation rights should be addressed to [email protected]. D/2011/0448/25 The following list contains the classification decisions (other than those subject to a reservation) taken by the Harmonized System Committee ( 47th Session – March 2011) on specific products, together with their related Harmonized System code numbers and, in certain cases, the classification rationale. Advice Parties seeking to import or export merchandise covered by a decision are advised to verify the implementation of the decision by the importing or exporting country, as the case may be. HS codes Classification No Product description Classification considered rationale 1. Preparation, in the form of a powder, consisting of 92 % sugar, 6 % 2106.90 GRIs 1 and 6 black currant powder, anticaking agent, citric acid and black currant flavouring, put up for retail sale in 32-gram sachets, intended to be consumed as a beverage after mixing with hot water. 2. Vanutide cridificar (INN List 100). 3002.20 3. Certain INN products. Chapters 28, 29 (See “INN List 101” at the end of this publication.) and 30 4. Certain INN products. Chapters 13, 29 (See “INN List 102” at the end of this publication.) and 30 5. Certain INN products. Chapters 28, 29, (See “INN List 103” at the end of this publication.) 30, 35 and 39 6. Re-classification of INN products. -

( 12 ) United States Patent
US010317418B2 (12 ) United States Patent ( 10 ) Patent No. : US 10 ,317 ,418 B2 Goosens (45 ) Date of Patent: * Jun . 11 , 2019 (54 ) USE OF GHRELIN OR FUNCTIONAL 7 , 479 ,271 B2 1 / 2009 Marquis et al . GHRELIN RECEPTOR AGONISTS TO 7 ,632 , 809 B2 12 / 2009 Chen 7 ,666 , 833 B2 2 /2010 Ghigo et al. PREVENT AND TREAT STRESS -SENSITIVE 7 , 901 ,679 B2 3 / 2011 Marquis et al . PSYCHIATRIC ILLNESS 8 ,013 , 015 B2 9 / 2011 Harran et al . 8 ,293 , 709 B2 10 /2012 Ross et al . (71 ) Applicant: Massachusetts Institute of 9 ,724 , 396 B2 * 8 / 2017 Goosens A61K 38 /27 9 , 821 ,042 B2 * 11 /2017 Goosens .. A61K 39/ 0005 Technology , Cambridge , MA (US ) 10 , 039 ,813 B2 8 / 2018 Goosens 2002/ 0187938 A1 12 / 2002 Deghenghi (72 ) Inventor : Ki Ann Goosens, Cambridge , MA (US ) 2003 / 0032636 Al 2 /2003 Cremers et al. 2004 / 0033948 Al 2 / 2004 Chen ( 73 ) Assignee : Massachusetts Institute of 2005 / 0070712 A1 3 /2005 Kosogof et al. Technology , Cambridge , MA (US ) 2005 / 0148515 Al 7/ 2005 Dong 2005 / 0187237 A1 8 / 2005 Distefano et al. 2005 /0191317 A1 9 / 2005 Bachmann et al. ( * ) Notice : Subject to any disclaimer , the term of this 2005 /0201938 A1 9 /2005 Bryant et al. patent is extended or adjusted under 35 2005 /0257279 AL 11 / 2005 Qian et al. U . S . C . 154 ( b ) by 0 days. 2006 / 0025344 Al 2 /2006 Lange et al. 2006 / 0025566 A 2 /2006 Hoveyda et al. This patent is subject to a terminal dis 2006 / 0293370 AL 12 / 2006 Saunders et al . -

VIII. Hypothalamo Pituitary Axis Bone Turnover
Drugs affecting the hypothalamic – pituitary axis Regina Demlová, FÚ LF MU The hypothalamo-pituitary axis is the unit formed by the hypothalamus and pituitary gland , which exerts control over many parts of the endocrine system. the nervous system regulates the endocrine system and endocrine activity modulates the activity of the CNS. Hormones (peptides) – receptors – muscles, bones, fat tissue A/ membrane R → AC – catalyze the conversion of ATP to cAMP and pyrophosphate - a regulatory signal via specific cAMP-binding proteins, either transcription factors or other enzymes (e.g., cAMP- dependent kinases). B/ cytosolic receptor → translocation to the nucleus in a form capable of interacting with the DNA. Controlling the hypothalamic-pituitary-target organ axis : . important factor Negative feedback the action of hypothalamic hormones may be inhibited : A/ by long feedback patway from the target gland hormone or B/ by short feedback pathway from the pituitary hormone. There may also be direct feedback from the target gland hormone to the pituitary gland. Input is also received at the hypothalamus from higher brain centres Negative feedback CNS (sensoric, psychogenic) HYPOTHALAMIC HORMONES Releasing factors Long negative „feedback“ pathway Short negative „feedback“ pathway PITUITARY GLAND „trophs“ TSH ACTH FSH LH peripheral endocrine tissue gland Endocrinopathy • any disease due to disorder of the endocrine system A. the hypothalamic-pituitary-target organ axis • Peripheral - primary - arise at the level of peripheral endocrine.glands -

Growth Hormone Secretagogues: History, Mechanism of Action and Clinical Development
Growth hormone secretagogues: history, mechanism of action and clinical development Junichi Ishida1, Masakazu Saitoh1, Nicole Ebner1, Jochen Springer1, Stefan D Anker1, Stephan von Haehling 1 , Department of Cardiology and Pneumology, University Medical Center Göttingen, Göttingen, Germany Abstract Growth hormone secretagogues (GHSs) are a generic term to describe compounds which increase growth hormone (GH) release. GHSs include agonists of the growth hormone secretagogue receptor (GHS‐R), whose natural ligand is ghrelin, and agonists of the growth hormone‐releasing hormone receptor (GHRH‐R), to which the growth hormone‐ releasing hormone (GHRH) binds as a native ligand. Several GHSs have been developed with a view to treating or diagnosisg of GH deficiency, which causes growth retardation, gastrointestinal dysfunction and altered body composition, in parallel with extensive research to identify GHRH, GHS‐R and ghrelin. This review will focus on the research history and the pharmacology of each GHS, which reached randomized clinical trials. Furthermore, we will highlight the publicly disclosed clinical trials regarding GHSs. Address for correspondence: Corresponding author: Stephan von Haehling, MD, PhD Department of Cardiology and Pneumology, University Medical Center Göttingen, Göttingen, Germany Robert‐Koch‐Strasse 40, 37075 Göttingen, Germany, Tel: +49 (0) 551 39‐20911, Fax: +49 (0) 551 39‐20918 E‐mail: [email protected]‐goettingen.de Key words: GHRPs, GHSs, Ghrelin, Morelins, Body composition, Growth hormone deficiency, Received 10 September 2018 Accepted 07 November 2018 1. Introduction testing in clinical trials. A vast array of indications of ghrelin receptor agonists has been evaluated including The term growth hormone secretagogues growth retardation, gastrointestinal dysfunction, and (GHSs) embraces compounds that have been developed altered body composition, some of which have received to increase growth hormone (GH) release. -

Restoring Youth
Restoring Youth The New Science of Human Growth Hormone Therapy Dr. Richard Gaines TABLE OF CONTENTS CHAPI'ER 1 Introduction to Human Growth 1 Hormone Replacement Therapy SECION 1 History of Growth Hormone Use 2 SECION 2 HGH and Hollywood 5 SECION 3 The Benefits of Growth Hormone 8 SECION 4 How HGH Levels are Tested 17 SECION 5 Using Growth Hormone 23 SECION 6 What are the Side Effects? 28 SECION 7 How Much does HGH Cost? 34 SECION 8 Buying Human Growth Hormone 38 SECION 9 Is HGH Legal? 44 CHAPI'ER 2 Related Hormones 47 SECION 1 Testosterone and HGH 48 SECION 2 Thyroid Hormone and HGH 53 SECION 3 Cortisol and Adrenal Fatigue 55 SECION 4 Insulin-like Growth Factor 57 SECION 5 GHRH and Sermorelin 60 SECION 6 Other Hormones and HGH 63 SECION 7 HGH Secretagogues 67 SECION 8 How to Boost HGH Naturally 74 CHAPI'ER 3 HGH Therapy for Men 79 SECION 1 Men and Comprehensive HRT 8o SECION 2 HGH Benefits for Men 86 SECION 3 HGH and Andropause 89 SECION 4 Adrenal Fatigue in Men 94 SECION 5 Men: Is HGH Right for You? 99 CHAPTER 4 HGH Therapy for Women 102 SECION 1 Women and Comprehensive HRT 103 SECION 2 HGH Benefits for Women 110 SECION 3 HGH and Menopause 114 SECION 4 Low Thyroid Syndrome in Women 122 SECION 5 Adrenal Fatigue in Women 128 SECION 6 Women and Bioidentical Hormones 131 SECION 7 Women: Is HGH Right for You? 136 SECION 8 One Woman's Story 139 CHAPTER 5 Choosing the Right HRT Provider 142 SECION 1 Things to Know 143 SECION 2 About HealthGAINS 150 SECION 3 FAQs Frequently Asked Questions 155 CHAPTER 6 Clinical Research 161 SECION 1 What Doctors Say 162 SECION 2 General Health 165 SECION 3 The Brain 168 SECION 4 Weight Loss 171 SECION 5 Anti-Aging 173 SECION 6 Article (Reprint) 175 Low T and Growth Hormone CHAPTER7 Product Information 179 SECION 1 FDA-Approved HGH 180 SECION 2 Illegal HGH 184 SECION 3 Related HGH Hormones 188 SECION 4 Rx HGH Peptides 193 SECION 5 HGH Oral Peptide Secretagogues 196 SECION 6 Homeopathic HGH 199 SECION 7 HGH Supplements 201 Preface THE CONTENT CONTAINED HEREIN IS MEANT TO BE INFORMATIVE ONLY. -

(12) Patent Application Publication (10) Pub. No.: US 2016/0243197 A1 G00 Sens (43) Pub
US 20160243 197A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0243197 A1 G00 sens (43) Pub. Date: Aug. 25, 2016 (54) USE OF GHRELIN ORFUNCTIONAL Publication Classification GHRELIN RECEPTORAGONSTS TO PREVENT AND TREAT STRESS-SENSITIVE (51) Int. Cl. PSYCHATRC LLNESS A638/22 (2006.01) GOIN33/74 (2006.01) (71) Applicant: Massachusetts Institute of Technology, A613 L/435 (2006.01) Cambridge, MA (US) (52) U.S. Cl. CPC ............... A61K 38/22 (2013.01); A61 K3I/435 (72) Inventor: Ki Ann Goosens, Cambridge, MA (US) (2013.01); G0IN33/74 (2013.01); G0IN (73) Assignee: Massachusetts Institute of Technology, 2800/7004 (2013.01); G0IN 2800/54 (2013.01); Cambridge, MA (US) G0IN 2333/575 (2013.01) (21) Appl. No.: 15/052,110 (57) ABSTRACT (22) Filed: Feb. 24, 2016 The invention relates to methods of treating stress-sensitive psychiatric diseases arising from trauma in a Subject by Related U.S. Application Data enhancing ghrelin signaling in the BLA of the Subject. The (60) Provisional application No. 62/119,898, filed on Feb. invention also relates to methods of reversing ghrelin resis 24, 2015. tance. Patent Application Publication Aug. 25, 2016 Sheet 1 of 18 US 2016/0243.197 A1 itediate Baseline Sarapie Auditory fear critioning Sarpie at 8, it, 36, 60, i28, atti i8 it long-term context long-terra Aisitory ear Recai Fair Recai F.G. 1A Patent Application Publication Aug. 25, 2016 Sheet 2 of 18 US 2016/0243.197 A1 10. a 30 s t 60 5 40 s { 200 t 3. 2 8 Minutes. -

2019 Prohibited List
THE WORLD ANTI-DOPING CODE INTERNATIONAL STANDARD PROHIBITED LIST JANUARY 2019 The official text of the Prohibited List shall be maintained by WADA and shall be published in English and French. In the event of any conflict between the English and French versions, the English version shall prevail. This List shall come into effect on 1 January 2019 SUBSTANCES & METHODS PROHIBITED AT ALL TIMES (IN- AND OUT-OF-COMPETITION) IN ACCORDANCE WITH ARTICLE 4.2.2 OF THE WORLD ANTI-DOPING CODE, ALL PROHIBITED SUBSTANCES SHALL BE CONSIDERED AS “SPECIFIED SUBSTANCES” EXCEPT SUBSTANCES IN CLASSES S1, S2, S4.4, S4.5, S6.A, AND PROHIBITED METHODS M1, M2 AND M3. PROHIBITED SUBSTANCES NON-APPROVED SUBSTANCES Mestanolone; S0 Mesterolone; Any pharmacological substance which is not Metandienone (17β-hydroxy-17α-methylandrosta-1,4-dien- addressed by any of the subsequent sections of the 3-one); List and with no current approval by any governmental Metenolone; regulatory health authority for human therapeutic use Methandriol; (e.g. drugs under pre-clinical or clinical development Methasterone (17β-hydroxy-2α,17α-dimethyl-5α- or discontinued, designer drugs, substances approved androstan-3-one); only for veterinary use) is prohibited at all times. Methyldienolone (17β-hydroxy-17α-methylestra-4,9-dien- 3-one); ANABOLIC AGENTS Methyl-1-testosterone (17β-hydroxy-17α-methyl-5α- S1 androst-1-en-3-one); Anabolic agents are prohibited. Methylnortestosterone (17β-hydroxy-17α-methylestr-4-en- 3-one); 1. ANABOLIC ANDROGENIC STEROIDS (AAS) Methyltestosterone; a. Exogenous* -

Volume 23, Issue 1, Winter 2016/2017
Neuroendocrine & Pituitary Tumor Clinical Center (NEPTCC) Bulletin WINTER 2016/2017 | VOLUME 23 | ISSUE 1 Understanding Growth Hormone Deficiency in HIV Lipodystrophy SUMAN SRINIVASA, MD Perturbations in the growth hormone (GH) axis are common among a significant role in improving metabolic outcomes, related to the well-treated human immunodeficiency virus (HIV)-infected improvements in body composition, dyslipidemia, inflammation, population [1]. Acquired GH deficiency (GHD) secondary to HIV is a cardiovascular and bone health [7-13], which could serve as an clinical diagnosis in the absence of known pituitary or hypothalamic important treatment paradigm in HIV lipodystrophy. pathology and should be differentiated from idiopathic adult Detailed physiology studies have been performed to characterize GH GHD. Studies have determined that a GH peak cutoff of 7.5 ng/mL dynamics in HIV. Overnight frequent sampling among HIV-infected during the GHRH-arginine test can adequately discriminate GHD individuals with and without lipodystrophy and healthy individuals in HIV-infected individuals compared to healthy individuals with demonstrated that the HIV group with lipodystrophy had reduced good specificity. As much as a third of HIV-infected individuals are basal and mean GH concentration, as well as GH pulse amplitude, estimated to have an inappropriate response to GHRH-arginine while GH pulse frequency and IGF-1 levels were normal when stimulation testing [2]. compared to the HIV non-lipodystrophy and healthy control groups A spectrum of disease from true GHD to GH insufficiency exists [14]. Visceral adipose tissue (VAT) accumulation was predictive of among the HIV population. Recent studies have reported that the reduced GH in the HIV population. -
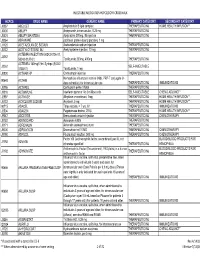
Injectable Medication Hcpcs/Dofr Crosswalk
INJECTABLE MEDICATION HCPCS/DOFR CROSSWALK HCPCS DRUG NAME GENERIC NAME PRIMARY CATEGORY SECONDARY CATEGORY J0287 ABELCET Amphotericin B lipid complex THERAPEUTIC INJ HOME HEALTH/INFUSION** J0400 ABILIFY Aripiprazole, intramuscular, 0.25 mg THERAPEUTIC INJ J0401 ABILIFY MAINTENA Apriprazole 300mg, IM injection THERAPEUTIC INJ J9264 ABRAXANE paclitaxel protein-bound particles, 1 mg J1120 ACETAZOLAMIDE SODIUM Acetazolamide sodium injection THERAPEUTIC INJ J0132 ACETYLCYSTEINE INJ Acetylcysteine injection, 10 mg THERAPEUTIC INJ ACTEMRA INJECTION (50242-0136-01, J3262 50242-0137-01) Tocilizumab 200mg, 400mg THERAPEUTIC INJ ACTEMRA 162mg/0.9ml Syringe (50242- J3262 SELF-INJECTABLE 0138-01) Tocilizumab, 1 mg J0800 ACTHAR HP Corticotropin injection THERAPEUTIC INJ Hemophilus influenza b vaccine (Hib), PRP-T conjugate (4- 90648 ACTHIB dose schedule), for intramuscular use THERAPEUTIC INJ IMMUNIZATIONS J0795 ACTHREL Corticorelin ovine triflutal THERAPEUTIC INJ J9216 ACTIMMUNE Interferon gamma 1-b 3 miillion units SELF-INJECTABLE CHEMO ADJUNCT* J2997 ACTIVASE Alteplase recombinant, 1mg THERAPEUTIC INJ HOME HEALTH/INFUSION** J0133 ACYCLOVIR SODIUM Acyclovir, 5 mg THERAPEUTIC INJ HOME HEALTH/INFUSION** 90715 ADACEL Tdap vaccine, > 7 yrs, IM THERAPEUTIC INJ IMMUNIZATIONS J2504 ADAGEN Pegademase bovine, 25 IU THERAPEUTIC INJ HOME HEALTH/INFUSION** J9042 ADCETRIS Brentuximab vedotin Injection THERAPEUTIC INJ CHEMOTHERAPY J0153 ADENOCARD Adenosine 6 MG THERAPEUTIC INJ J0171 ADRENALIN Adrenalin (epinephrine) inject THERAPEUTIC INJ J9000 ADRIAMYCIN Doxorubicin -

Safety and Metabolic Effects of Tesamorelin, a Growth Hormone-Releasing Factor Analogue, in Patients with Type 2 Diabetes: a Randomized, Placebo-Controlled Trial
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Carolina Digital Repository RESEARCH ARTICLE Safety and metabolic effects of tesamorelin, a growth hormone-releasing factor analogue, in patients with type 2 diabetes: A randomized, placebo-controlled trial David R. Clemmons1*, Sam Miller2, Jean-Claude Mamputu3 1 Division of Endocrinology, Department of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, a1111111111 North Carolina, United States of America, 2 SAM Clinical Research Center, San Antonio, Texas, United States of America, 3 Theratechnologies Inc., Montreal, Quebec, Canada a1111111111 a1111111111 * [email protected] a1111111111 a1111111111 Abstract OPEN ACCESS Objective Citation: Clemmons DR, Miller S, Mamputu J-C Use of growth hormone is associated with side effects, including insulin resistance. The (2017) Safety and metabolic effects of tesamorelin, objective of this study was to determine whether tesamorelin, a stabilized growth hormone- a growth hormone-releasing factor analogue, in patients with type 2 diabetes: A randomized, releasing hormone analogue, would alter insulin sensitivity or control of diabetes. placebo-controlled trial. PLoS ONE 12(6): e0179538. https://doi.org/10.1371/journal. pone.0179538 Design Editor: Noel Christopher Barengo, Florida A 12-week randomized, placebo-controlled study of 53 patients with type 2 diabetes. Three International University Herbert Wertheim College treatment groups: placebo, 1 and 2 mg tesamorelin. of Medicine, UNITED STATES Received: September 7, 2016 Measurements Accepted: May 25, 2017 Fasting glucose, glucose and insulin from oral glucose tolerance test, glycosylated hemo- Published: June 15, 2017 globin (HbA1c), home blood glucose, insulin-like growth factor-1, and lipids.