Health Effects of Interactions Between Tobacco Use and Exposure to Other Agents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Project SUN: a Study of the Illicit Cigarette Market In
Project SUN A study of the illicit cigarette market in the European Union, Norway and Switzerland 2017 Results Executive Summary kpmg.com/uk Important notice • This presentation of Project SUN key findings (the ‘Report’) has been prepared by KPMG LLP the UK member firm (“KPMG”) for the Royal United Services Institute for Defence and Security Studies (RUSI), described in this Important Notice and in this Report as ‘the Beneficiary’, on the basis set out in a private contract dated 27 April 2018 agreed separately by KPMG LLP with the Beneficiary (the ‘Contract’). • Included in the report are a number of insight boxes which are written by RUSI, as well as insights included in the text. The fieldwork and analysis undertaken and views expressed in these boxes are RUSI’s views alone and not part of KPMG’s analysis. These appear in the Foreword on page 5, the Executive Summary on page 6, on pages 11, 12, 13 and 16. • Nothing in this Report constitutes legal advice. Information sources, the scope of our work, and scope and source limitations, are set out in the Appendices to this Report. The scope of our review of the contraband and counterfeit segments of the tobacco market within the 28 EU Member States, Switzerland and Norway was fixed by agreement with the Beneficiary and is set out in the Appendices. • We have satisfied ourselves, so far as possible, that the information presented in this Report is consistent with our information sources but we have not sought to establish the reliability of the information sources by reference to other evidence. -
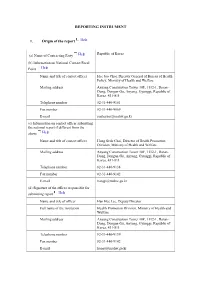
REPORTING INSTRUMENT 1. Origin of the Report
REPORTING INSTRUMENT 1. Origin of the report Help Question 1 is mandatory Republic of Korea (a) Name of Contracting Party Help (b) Information on National Contact/Focal Point Help Name and title of contact officer Hee Joo Choi, Director General of Bureau of Health Policy, Ministry of Health and Welfare Mailing address Anyang Construction Tower 10F, 1112-1, Daran- Dong, Dongan-Gu, Anyang, Gyunggi, Republic of Korea, 431-811 Telephone number 82-31-440-9101 Fax number 82-31-440-9069 E-mail [email protected] (c) Information on contact officer submitting the national report if different from the above Help Name and title of contact officer Hong Seok Choi, Director of Health Promotion Division, Ministry of Health and Welfare Mailing address Anyang Construction Tower 10F, 1112-1, Daran- Dong, Dongan-Gu, Anyang, Gyunggi, Republic of Korea, 431-811 Telephone number 82-31-440-9138 Fax number 82-31-440-9142 E-mail [email protected] (d) Signature of the officer responsible for submitting report Help Name and title of officer Han Hee Lee, Deputy Director Full name of the institution Health Promotion Division, Ministry of Health and Welfare Mailing address Anyang Construction Tower 10F, 1112-1, Daran- Dong, Dongan-Gu, Anyang, Gyunggi, Republic of Korea, 431-811 Telephone number 82-31-440-9139 Fax number 82-31-440-9142 E-mail [email protected] Web page 15/8/2005-15/8/2007 (e) Period reported Help 31/8/2007 (f) Date the report was submitted Help 2. Demographics Help Create Age Group (a) Age and sex: Help Question 2(a) is mandatory Year Percentage of Percentage Percentage of Age groups male of female total (latest available) population population population 2005 13-15 4.37 3.89 4.13 2005 16-17 2.66 2.44 2.55 2005 19-24 9.39 8.85 9.12 2005 25-34 17.32 16.52 16.92 2005 35-44 17.90 17.33 17.62 2005 45-54 14.28 14.09 14.18 2005 55-64 8.44 9.00 8.72 2005 65+ 7.17 10.99 9.07 Create Ethnic Group (b) Ethnicity (optional): Help Question 2(b) is optional Name of ethnic group Percentage of total population 3. -

Treasury and General Government Appropriations for Fiscal Year 2001
S. HRG. 106±712 TREASURY AND GENERAL GOVERNMENT APPROPRIATIONS FOR FISCAL YEAR 2001 HEARINGS BEFORE A SUBCOMMITTEE OF THE COMMITTEE ON APPROPRIATIONS UNITED STATES SENATE ONE HUNDRED SIXTH CONGRESS SECOND SESSION ON H.R. 4871/S. 2900 AN ACT MAKING APPROPRIATIONS FOR THE TREASURY DEPARTMENT, THE UNITED STATES POSTAL SERVICE, THE EXECUTIVE OFFICE OF THE PRESIDENT, AND CERTAIN INDEPENDENT AGENCIES FOR THE FISCAL YEAR ENDING SEPTEMBER 30, 2001, AND FOR OTHER PUR- POSES Department of the Treasury Executive Office of the President Nondepartmental witnesses Printed for the use of the Committee on Appropriations ( Available via the World Wide Web: http://www.access.gpo.gov/congress/senate U.S. GOVERNMENT PRINTING OFFICE 62±810 cc WASHINGTON : 2000 For sale by the U.S. Government Printing Office Superintendent of Documents, Congressional Sales Office, Washington, DC 20402 COMMITTEE ON APPROPRIATIONS TED STEVENS, Alaska, Chairman THAD COCHRAN, Mississippi ROBERT C. BYRD, West Virginia ARLEN SPECTER, Pennsylvania DANIEL K. INOUYE, Hawaii PETE V. DOMENICI, New Mexico ERNEST F. HOLLINGS, South Carolina CHRISTOPHER S. BOND, Missouri PATRICK J. LEAHY, Vermont SLADE GORTON, Washington FRANK R. LAUTENBERG, New Jersey MITCH MCCONNELL, Kentucky TOM HARKIN, Iowa CONRAD BURNS, Montana BARBARA A. MIKULSKI, Maryland RICHARD C. SHELBY, Alabama HARRY REID, Nevada JUDD GREGG, New Hampshire HERB KOHL, Wisconsin ROBERT F. BENNETT, Utah PATTY MURRAY, Washington BEN NIGHTHORSE CAMPBELL, Colorado BYRON DORGAN, North Dakota LARRY CRAIG, Idaho DIANNE FEINSTEIN, California KAY BAILEY HUTCHISON, Texas RICHARD J. DURBIN, Illinois JON KYL, Arizona STEVEN J. CORTESE, Staff Director LISA SUTHERLAND, Deputy Staff Director JAMES H. ENGLISH, Minority Staff Director SUBCOMMITTEE ON TREASURY AND GENERAL GOVERNMENT BEN NIGHTHORSE CAMPBELL, Colorado, Chairman RICHARD C. -

TOBACCO CONTROL GOVERNANCE in SUB-SAHARAN AFRICA Advance Copy February 2016
Empowered lives. TOBACCO Resilient nations. CONTROL GOVERNANCE IN SUB-SAHARAN AFRICA Implementing Article 5.2(a) of the World Health Organization Framework Convention on Tobacco Control Advance Copy February 2016 DISCUSSION PAPER Empowered lives. TOBACCO Resilient nations. CONTROL GOVERNANCE IN SUB-SAHARAN AFRICA Implementing Article 5.2(a) of the World Health Organization Framework Convention on Tobacco Control Advance Copy February 2016 DISCUSSION PAPER Empowered lives. TOBACCO Resilient nations. CONTROL GOVERNANCE IN SUB-SAHARAN AFRICA Implementing Article 5.2(a) of the World Health Organization Framework Convention on Tobacco Control Advance Copy February 2016 DISCUSSION PAPER TOBACCO CONTROL GOVERNANCE IN SUB-SAHARAN AFRICA Advance Copy February 2016 TABLE OF CONTENTS Acknowledgements ����������������������������������������������������������������������������������������������������������������������������������������������������������������2 Abbreviations ���������������������������������������������������������������������������������������������������������������������������������������������������������������������������3 Executive summary ��������������������������������������������������������������������������������������������������������������������������������������������������������������� 4 Structure of the document ������������������������������������������������������������������������������������������������������������������������������������������������������6 Chapter 1 – Background ��������������������������������������������������������������������������������������������������������������������������������������������������������7 -

Inquiry Into the Use and Marketing of Electronic Cigarettes and Personal Vaporisers in Australia, July 2017 – Submission by Fact Asia Consultants Ltd (“Factasia.Org”)
Standing Committee on Health, Aged Care & Sport: Inquiry into the Use and Marketing of Ele troni Cigarettes and !ersonal "aporisers in Australia, #uly $%&' ( submission by *a t Asia Consultants +td ,-fa tasia.org”0 As an independent regional consumer advocacy with considerable experience of the important issue of tobacco harm reduction and in particular the often contentious debate over 'vaping', factasia.org welcomes the opportunity to submit comment on the current situation regarding the availability and regulatory treatment in Australia of alternative nicotine products. factasia.org is an independent, not-for-profit, consumer-oriented advocate for rational debate about – and sensible regulation of – the rights of adult citizens throughout Asia to choose for themselves within sensibly defined guidelines. factasia is currently concentrating on the issue of consumers' ability to choose tobacco harm reduction in numerous countries across Asia-Pacific including Hong Kong (with presentations to the Legislative Council), Malaysia (discussions with Minister of Health and officials at the Trade Ministry) and the Philippines, as well as Australia and New Zealand. factasia does not support smoking or promote the use of nicotine, opposes all under-age use of personal vaporisers (with or without nicotine) or any other new nicotine product, and does not (and will not) engage in any manufacturing, distribution or retailing activities. factasia aims to act as a messenger, facilitating constructive dialogue between scientists and medical experts, legislators, regulators and the general public. The last year has seen several in-depth analyses of the science and medicine involved in the arguments, not least with Australia's TGA and its consultation exercise. -

The Impact of Tobacco Control Research on Policy: 20 Years of Progress Kenneth E Warner, Jamie Tam
The tobacco epidemic today Tob Control: first published as 10.1136/tobaccocontrol-2011-050396 on 16 February 2012. Downloaded from The impact of tobacco control research on policy: 20 years of progress Kenneth E Warner, Jamie Tam Department of Health ABSTRACT (2) to examine how research and policy have Management & Policy, School of Objectives To assess progress in tobacco control policy interacted and influenced each other. We address Public Health, University of research and the relevance of research to policymaking. the first objective by presenting findings from Michigan, Michigan, USA Methods Over 100 experts were surveyed about their a survey of tobacco control experts regarding Correspondence to opinions on the body of research existing in 1992 and progress in research over the past two decades on Professor Kenneth E Warner, 2011 concerning 11 areas of tobacco control policy, the a series of core policy areas. We also consider Department of Health state of policy implementation in both years, the extent respondents’ assessments of progress in the adop- Management & Policy, School of to which research has affected policy adoption and how tion and implementation of these policies, the role Public Health, University of fl Michigan, 1415 Washington experience with policy has influenced research. Case of research in in uencing policy adoption and how Heights, Ann Arbor, Michigan, studies of how research and policy implementation have policy experience can influence research. We then USA; [email protected] interacted were developed. consider the often-complicated relationships Results The body of research was not judged between research and policy in three policy Received 16 August 2011 Accepted 19 December 2011 ‘substantial’ in any of the policy areas in 1992. -

2020 2020 - Core Questionnaire of the Reporting Instrument of Who Fctc
8/29/2020 2020 - CORE QUESTIONNAIRE OF THE REPORTING INSTRUMENT OF WHO FCTC 2020 - CORE QUESTIONNAIRE OF THE REPORTING INSTRUMENT OF WHO FCTC A. ORIGIN OF THE REPORT Name of contracting Party: Republic of Korea Information on national contact responsible for preparation of the report: Title Deputy Director, Bureau of Health Policy Family name JEON First name GAEUN Full name of institution Ministry of Health and Welfare Mailing address Mailing address 1 13 Doum 4-ro Mailing address 2 Post code 30113 Post box City Sejong Country Republic of Korea E-mail [email protected] https://extranet.who.int/dataformv3/index.php/plugins/direct?plugin=AdminPrintAnswer&function=printAnswer&surveyid=829214&srid=64&lang= 1/64 8/29/2020 2020 - CORE QUESTIONNAIRE OF THE REPORTING INSTRUMENT OF WHO FCTC Alternative email address Telephone number +82 44 202 2822 Fax number +82 44 202 3938 Signature of government official submitting the report: Title Deputy Director, Bureau of Health Policy Family name JEON First name GAEUN Full name of institution Ministry of Health and Welfare Mailing address Mailing address 1 13 Doum 4-ro Mailing address 2 Post code 30113 Post box City Sejong Country Republic of Korea E-mail [email protected] Alternative email address Telephone number +82 44 202 2826 https://extranet.who.int/dataformv3/index.php/plugins/direct?plugin=AdminPrintAnswer&function=printAnswer&surveyid=829214&srid=64&lang= 2/64 8/29/2020 2020 - CORE QUESTIONNAIRE OF THE REPORTING INSTRUMENT OF WHO FCTC Fax number +82 44 202 3938 Web page www.mohw.go.kr Period of reporting: Month Year Start date April (4) 2018 (19) End date March (3) 2020 (21) B1. -

(2004) Report on Tobacco Control in India
Report on Tobacco Control in India Edited by K. Srinath Reddy Prakash C. Gupta This report is jointly supported by Ministry of Health & Family Welfare, Government of India Centers for Disease Control and Prevention, USA World Health Organization Tobacco Control in India Report on Tobacco Control in India (New Delhi, India), 25 November 2004 Ministry of Health & Family Welfare, Nirman Bhawan, Maulana Azad Road, New Delhi 110011, India Disclaimer: The views expressed in this report are not necessarily those of the Ministry of Health & Family Welfare, Government of India, who commissioned the report as well as the World Health Organization and Centers for Disease Control and Prevention (USA), who provided technical guidance. Preparation of this report has been jointly undertaken by HRIDAY, New Delhi, India and Tata Memorial Centre, Mumbai, India HRIDAY Tata Memorial Centre T-7, Green Park Extension Dr Ernest Borges Marg, Parel New Delhi 110016 Mumbai 400012 India up to 31 July 2004; since then Healis Sekhsaria Institute of Public Health 601, Great Eastern Chambers 6th Floor, Plot No. 28, Sector 11 CBD Belapur (E) Navi Mumbai 400614 India on behalf of Ministry of Health & Family Welfare, Government of India The report has been technically edited by BYWORD EDITORIAL CONSULTANTS A-217, Somdatt Chambers I, Bhikaiji Cama Place New Delhi 110016, India Printed at Shree Om Enterprises Pvt. Ltd., A-98/3 Okhla Industrial Area, Pahse II, New Delhi 110020 ii Tobacco Control in India Foreword Hkkjr ljdkj LokLF; ,oa ifjokj dY;k.k ea=ky; ubZ fnYyh & 110011 GOVERNMENT OF INDIA MINISTRY OF HEALTH & FAMILY WELFARE NEW DELHI - 110011 J.V.R. -

Fulstop FAITH-BASED ORGANIZATIONS RESOURCE
FULSToP FAMILIES UNITING LOCALLY TO SOLVE TOBACCO PROLIFERATION FAITH-BASED ORGANIZATIONS RESOURCE PACKET 2 | P a g e TABLE OF CONTENTS I. INTRODUCTION Overview of Tobacco-Free Policy for Faith-Based Organizations 3 Tobacco Free Model Policy 4 Tobacco Free Model Signage 7 Directory of Faith Based Organizations with Adopted Policies 8 II. RESOURCES Tobacco Timeline 9 Story of Inequity: African Americans 11 Protect the Health of Your Congregation 15 The Truth About Flavored Tobacco 21 The Truth About Menthol Cigarettes 23 Secondhand Smoking in Housing 25 Thirdhand Smoke- Protecting your Family 26 FULSToP contract 17-10977 receives funds from the California Department of Public Health, California Tobacco Control Program, through the Proposition 56 California Healthcare, Research, and Prevention Tobacco Act of 2016. 8.31.20 3 | P a g e Overview of Tobacco-Free Policy for Faith-Based Organizations Purpose Statement According to the U.S. Surgeon General’s Report of 2006, the Environmental Protection Agency of 1992 and the Federal Pro-Children Act of 1994, tobacco use and exposure to secondhand smoke (environmental tobacco smoke) are hazardous to the health of human beings. Faith-based organizations can improve the health and well-being of congregational members and community residents. Tobacco in any form is a a major cause of preventable disease and death in California and the United States. It has also been acknowledged as a fire hazard. Thus, prohibiting tobacco products and electronic smoking devices at faith-based facilities, vehicles, and sponsored events, rejecting financial sponsorship from tobacco companies and eliminating tobacco advertising on gear or other paraphernalia at any function/activity, and adopting a written tobacco-free congregational policy helps to provide a safe and healthy environment for all people. -

2019 Full Year Report
Illicit tobacco in New Zealand 2019 Full Year Report 26 May 2020 kpmg.com/uk KPMG LLP Tel +44 (0)20 7311 1000 Strategy Group Fax +44 (0)20 7311 3311 15 Canada Square DX 157460 Canary Wharf 5 Canary Wharf London E14 5GL United Kingdom 26 May 2020 Important notice This presentation of key findings (the ‘Report’) has been prepared by KPMG LLP in the UK (‘KPMG UK’) for Imperial Tobacco New Zealand Limited, described in this Important Notice and in this Report as the ‘Beneficiary’, on the basis set out in a private contract dated 1st August 2019 agreed separately with the Beneficiary. Nothing in this Report constitutes legal advice. Information sources, the scope of our work, and scope and source limitations, are set out in the Appendices to this Report. The scope of our review of the contraband, counterfeit and unbranded segments of the tobacco market within New Zealand was fixed by agreement with the Beneficiary and is set out in the Appendices. We have satisfied ourselves, so far as possible, that the information presented in this Report is consistent with our information sources but we have not sought to establish the reliability of the information sources by reference to other evidence. This Report has not been designed to benefit anyone except the Beneficiary. In preparing this Report we have not taken into account the interests, needs or circumstances of anyone apart from the Beneficiary, even though we have been aware that others might read this Report. This Report is not suitable to be relied on by any party wishing to acquire rights or assert any claims against KPMG LLP (other than the Beneficiary) for any purpose or in any context. -

Border Integrity, Illicit Tobacco, and Canada's
BORDER INTEGRITY, ILLICIT TOBACCO, AND CANADA’S SECURITY Jean Daudelin with Stephanie Soiffer and Jeff Willows NATIONAL SECURITY STRATEGY 4 FOR CANADA SERIES 1 Andrew Graham Canada's CriticalCanada's Infrastructure: Critical When Infrastructure: is Safe Enough When Safeis Safe Enough? Enough Safe Enough Andrew Graham 1 True North in Canadian Public Policy Board of Directors Advisory Council CHAIR Purdy Crawford Rob Wildeboer Former CEO, Imasco, Counsel at Osler Hoskins Chairman, Martinrea International Inc., Toronto Jim Dinning Former Treasurer of Alberta MANAGING DIRECTOR Brian Lee Crowley Don Drummond Former Clifford Clark Visiting Economist Economics Advisor to the TD Bank, Matthews Fellow in at Finance Canada Global Policy and Distinguished Visiting Scholar at the School of Policy Studies at Queen’s University SECRETARY Brian Flemming Lincoln Caylor International lawyer, writer and policy advisor Partner, Bennett Jones, Toronto Robert Fulford Former editor of Saturday Night magazine, columnist TREASURER with the National Post, Toronto Martin MacKinnon CFO, Black Bull Resources Inc., Halifax Calvin Helin Aboriginal author and entrepreneur, Vancouver DIRECTORS Hon. Jim Peterson John Beck Former federal cabinet minister, Partner at Chairman and CEO, Aecon Construction Ltd., Fasken Martineau, Toronto Toronto Maurice B. Tobin Erin Chutter The Tobin Foundation, Washington DC President and CEO, Puget Ventures Inc., Vancouver Navjeet (Bob) Dhillon Research Advisory Board CEO, Mainstreet Equity Corp., Calgary Janet Ajzenstat Keith Gillam Professor -

British American Tobacco and Cigarette Smuggling in Asia J Collin, E Legresley, R Mackenzie, S Lawrence, K Lee
ii104 Tob Control: first published as 10.1136/tc.2004.009357 on 24 November 2004. Downloaded from RESEARCH PAPER Complicity in contraband: British American Tobacco and cigarette smuggling in Asia J Collin, E LeGresley, R MacKenzie, S Lawrence, K Lee ............................................................................................................................... Tobacco Control 2004;13(Suppl II):ii104–ii111. doi: 10.1136/tc.2004.009357 Objectives: To examine the complicity of British American Tobacco (BAT) in cigarette smuggling in Asia, See end of article for and to assess the centrality of illicit trade to regional corporate strategy. authors’ affiliations Methods: Analysis of previously confidential documents from BAT’s Guildford depository. An iterative ....................... strategy combined searches based on geography, organisational structure, and key personnel, while Correspondence to: corporate euphemisms for contraband were identified by triangulation. Dr Jeff Collin, Centre on Results: BAT documents demonstrate the strategic importance of smuggling across global, regional, Global Change and Health, London School of national, and local levels. Particularly important in Asia, contraband enabled access to closed markets, Hygiene and Tropical created pressure for market opening, and was highly profitable. Documents demonstrate BAT’s detailed Medicine, Keppel Street, oversight of illicit trade, seeking to reconcile the conflicting demands of control and deniability. London WC1E 7HT, UK; Conclusions: BAT documents demonstrate