Functional Genomics Workshop
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Autumn 2016 Meeting Booklet
Welcome Message from the ISR President Dr Sandy Fraser Dear Colleagues and Friends I have great pleasure in welcoming you all to The Killashee Hotel Naas for this year’s ISR Autumn Meeting. The programme for this year’s autumn meeting has been put together by our colleagues in Beaumont hospital, Dr Donough Howard, Dr Grainne Kearns and Dr Paul O’Connell and I think you will agree that it is a fascinating agenda addressing topics which are of interest to all of us. I look forward with great interest to hear the thoughts of Professor Dennis McGonagle , Professor of Investigative Rheumatology, Leeds Institute of Rheumatic and Musculoskeletal Medicine and indeed Dr Bruce Kirkham, consultant Rheumatologist at Guys and St. Thomas’ NHS Foundation Trust. Professor Sean Gaine from The Mater Misericordiae Hopital Dublin will present on the current management of pulmonary hypertension and Dr. Patrick Kiely from St Georges Hospital London will help us to pick the right biologic for our patients. Finally Professor Donal O’Shea from St. Vincents Hospital Dublin will address the issue of obesity and its relationship to inflammation. During the year the work of the ISR has continued unabated and Professor David Kane, as National Programme Director, has continued to develop the National Rheumatology Programme with consensus and inclusivity core to the process. The steering group (CAG – Clinical Advisory Group) for the programme is open to all members of the ISR and the last meeting at the spring meeting in Cork was very well attended and very productive and there was a great deal of agreement regarding the direction the programme should lead. -

MEDICALBJOURNAL Birthis, MARRIAGES, and DEATHS
1558 DEC. 28, 1957 MEDICAL NEWS MEDICALBJOURNAL aged sick, and £25.000 for grants to convalescent homes. Van Meter Prize.-The American Goiter Association Sir ARCHIBALD GRAY said the distribution committee had again offers the Van Meter Prize Award of $300 and two made a grant of £10,000 to the British Student Tuberculosis honourable mentions for the best essays on originial work Foundation. This Foundation had been started, he said, on problems related to the thyroid gland. Essays may cover entirely by university students, who since 1950 had collected either clinical or research investigations, should not exceed something like £35,000 to help students with pulmonary 3,000 words in length, and must be presented in English. tuberculosis. Up to the present, accommodation had been Duplicate typewritten copies, double spaced, should be sent in two centres outside London, to which it was difficult for to Dr. JOHN C. MCCLINTOCK, 149}, Washington Avenue, tutors from the various colleges to travel. Mottingham Albany, 10, New York, not later than February 1. Hall, in the grounds of Grove Park Hospital, Lewisham, Medical Auxiliaries.-Recognition was granted by the was now to be converted into a hostel, taking about 35 Board of Registration during 1956-7 to the Society of ambulant cases, and there would be two wards in Grove Audiology Technicians and the Institute of Technicians in Park Hospital for those who had to be hospitalized. The Venereology, it is stated in the Board's annual report. students would thus get much better accommodation, with During the year registers of orthoptists, operating theatre a resident warden, and be within easy reach of the various technicians, and dispensing opticians were published. -

Ardthe EULAR Journal Editor Josef S Smolen (Austria) and Disorders of Connective Tissue
Annals of the Rheumatic Diseases publishes original work on all aspects of rheumatology ARDThe EULAR Journal Editor Josef S Smolen (Austria) and disorders of connective tissue. Laboratory Associate Editors Tristan Barber (UK) and clinical studies are equally welcome Francis Berenbaum (France) Dimitrios Boumpas (Greece) Editorial Board Gerd Burmester (Germany) Daniel Aletaha (Austria) Robert Landewé (The Netherlands) Mary Crow (USA) Johan Askling (Sweden) Rik Lories (Belgium) Contact Details Iain McInnes (UK) Sang-Cheol Bae (Korea) Ingrid Lundberg (Sweden) Thomas Pap (Germany) Xenofon Baraliakos (Germany) Gary MacFarlane (UK) Editorial Office David Pisetsky (USA) Anne Barton (UK) Xavier Mariette (France) Annals of the Rheumatic Diseases Maarten Boers (The Alberto Martini (Italy) Désirée van der Heijde BMJ Journals, BMA House, Tavistock Square (The Netherlands) Netherlands) Dennis McGonagle (UK) London WCIH 9JR,UK Kazuhiko Yamamoto (Japan) Matthew Brown (Australia) Fred Miller (USA) Maya Buch (UK) Peter Nash (Australia) E: [email protected] Methodological and Statistical Loreto Carmona (Spain) Michael Nurmohamed (The Advisor Carlo Chizzolini (Switzerland) Netherlands) Production Editor Stian Lydersen (Norway) Bernard Combe (France) Caroline Ospelt (Switzerland) Teresa Jobson Philip Conaghan (UK) Monika Østensen (Norway) Social Media Editor E: [email protected] Harrison Austin (UK) Maurizio Cutolo (Italy) Constatino Pitzalis (UK) José da Silva (Portugal) Jane Salmon (USA) Social Media Advisors Nicola Dalbeth (Australia) Georg Schett (Germany) -

Social Media & Medicine Focus On
Fall 2013, Volume 23, Number 3 The Journal of the Canadian Rheumatology Association Focus on Social Media & Medicine Editorial • Freedom 55 Awards, Appointments, Regional News and Accolades • Rheumatology in Saint John, New Brunswick • Celebrating Dr. Robert Inman, Mr. Jean Légaré, • Greetings from Moncton Dr. Diane Lacaille, Mr. Daniel Longchamps, Dr. Dianne Mosher, and Dr. Rachel Shupak Joint Communiqué • EULAR 2013 Impression & Opinion • Canadian Rheumatology Association / Mexican • Using EMRs to Connect with Patients in Daily College of Rheumatology Update: 2013 Rheumatology Clinical Practice • Utilization of Musculoskeletal Ultrasound in Daily • Use of Technology and Medico-Legal Issues Rheumatology Practice and Research • Rhediant Diagnostic Software • Communicating With Seniors: A Workshop for • Mobile Apps For Your Practice Nursing Staff • RheumInfo.com • Some Thoughts on Social Media In Memoriam • Patient Communication via MyDoctor.ca • James Richard Topp Northern (High)Lights Hallway Consult • Abraham Shore Memorial Lectureship • If It’s Not One Thing, It’s Another: Transformation • Rheumatology Choosing Wisely of Lupus Nephritis What Is the CRA Doing For You? Top Ten Things Rheumatologists Should (And Might Not) Know About... • RA Guidelines: Practice Patterns of Rheumatologists in Canada Compared to CRA • Pain Recommendations for RA (Part II) The CRAJ is online! You can find us at: www.stacommunications.com/craj.html EDITORIAL Freedom 55 By Philip A. Baer, MDCM, FRCPC, FACR “Give me your answer, fill in a form Mine for evermore Will you still need me, will you still feed me When I'm sixty-four?” - Lennon-McCartney, “When I’m 64”, Sgt. Pepper’s Lonely Hearts Club Band , 1966. itting in my office, seeing a steady stream of patients, And here I thought I was doing particularly well with my I feel a gratifying sense of control and mastery most chronic inflammatory disease patients. -

Dr Barbara Mary Ansell
Archives of Disease in Childhood 1997;77:279–280 279 Arch Dis Child: first published as 10.1136/adc.77.4.279 on 1 October 1997. Downloaded from ARCHIVES OF DISEASE IN CHILDHOOD The Journal of the Royal College of Paediatrics and Child Health James Spence Medallist 1997 Dr Barbara Mary Ansell At the first Annual Meeting of the Royal College of Paedi- atrics and Child Health, in April at the University of York, the President, Professor Sir Roy Meadow, presented the James Spence Medal to Dr Barbara Ansell, with this citation. 1997 is the 100th Anniversary of George Frederic Still’s description of chronic joint disease in children. At least seven diVerent forms of idiopathic arthritis that commence in childhood are now recognised, and no-one has done more than today’s James Spence Medallist in defining these disorders and improving their management. Dr Barbara Ansell fulfils to the limit the criteria by which our premier award, the James Spence Medal, is awarded—for outstand- ing contributions to the advancement or clarification of http://adc.bmj.com/ paediatric knowledge. Barbara Ansell was educated at King’s High School for Girls in Warwick before entering, as a medical student, Birmingham University, from which she qualified in 1946. Thus, she has experienced the first 50 years of the National Health Service, and in many ways she exemplifies all that has been best about our health service in its first 50 years—the development of specialty services and, in on October 1, 2021 by guest. Protected copyright. particular, specialty services for children, the development of child and family centred services, the advent of clinical trials, the delivery of care for chronic disorders by multidisciplinary teams, and the availability of expert care on an equitable basis, and (though I hope it does not sound patronising) one of the most important aspects of today’s health service, the stature and role of women doctors, for whom opportunities previously were so limited. -

Early Development of Total Hip Replacement
EARLY DEVELOPMENT OF TOTAL HIP REPLACEMENT The transcript of a Witness Seminar held by the Wellcome Trust Centre for the History of Medicine at UCL, London, on 14 March 2006 Edited by L A Reynolds and E M Tansey Volume 29 2006 ©The Trustee of the Wellcome Trust, London, 2007 First published by the Wellcome Trust Centre for the History of Medicine at UCL, 2007 The Wellcome Trust Centre for the History of Medicine at UCL is funded by the Wellcome Trust, which is a registered charity, no. 210183. ISBN 978 085484 111 0 All volumes are freely available online at: www.history.qmul.ac.uk/research/modbiomed/wellcome_witnesses/ Please cite as: Reynolds L A, Tansey E M. (eds) (2007) Early Development of Total Hip Replacement. Wellcome Witnesses to Twentieth Century Medicine, vol. 29. London: Wellcome Trust Centre for the History of Medicine at UCL. CONTENTS Illustrations and credits v Abbreviations ix Witness Seminars: Meetings and publications; Acknowledgements E M Tansey and L A Reynolds xi Introduction Francis Neary and John Pickstone xxv Transcript Edited by L A Reynolds and E M Tansey 1 Appendix 1 Notes on materials by Professor Alan Swanson 95 Appendix 2 Surgical implant material standards by Mr Victor Wheble 97 Appendix 3 Selected prosthetic hips 101 References 107 Biographical notes 133 Glossary 147 Index 155 ILLUSTRATIONS AND CREDITS Figure 1 Site of a total hip transplant. Illustration provided by Ms Clare Darrah. 4 Figure 2 Mr Philip Wiles FRCS, c. 1950. Illustration provided by Sir Rodney Sweetnam. 5 Figure 3 X-ray of Wiles’ hip, c. -

Pediatric Rheumatology Biomed Central
Pediatric Rheumatology BioMed Central Commentary Open Access A peripatetic pediatrician's journey into pediatric rheumatology: Part II Earl J Brewer* Email: Earl J Brewer* - [email protected] * Corresponding author Published: 21 June 2007 Received: 19 June 2007 Accepted: 21 June 2007 Pediatric Rheumatology 2007, 5:14 doi:10.1186/1546-0096-5-14 This article is available from: http://www.ped-rheum.com/content/5/1/14 © 2007 Brewer; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Earl Brewer discusses his journey into pediatric rheumatology from 1958 to retirement in 1990 in three parts. Part II: The PRCSG and the Park City meetings in children. I conducted the study at the Ben Taub Hospi- in the US tal in Houston. It was published in Arthritis and Rheuma- IV Pediatric Rheumatology Collaborative Study Group tism in 1968. The study was able to show that (PRSCG) – 1973 indomethocin was superior to acetaminophen in control- The beginning of the PRSCG was part of the evolving plan ling fever. This was a short usage study, but no adverse for the future of pediatric rheumatology in the US. I was reactions occurred in the short dosage regimen. This impressed with the work of Maxwell Finland in Boston in study, however, did not address the problem of obtaining an earlier era when he cobbled together a group of clini- approval for the use of indomethacin in children. -
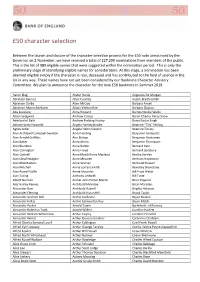
50 Character Selection
£50 character selection Between the launch and closure of the character selection process for the £50 note announced by the Governor on 2 November, we have received a total of 227,299 nominations from members of the public. This is the list of 989 eligible names that were suggested within the nomination period. This is only the preliminary stage of identifying eligible names for consideration: At this stage, a nomination has been deemed eligible simply if the character is real, deceased and has contributed to the field of science in the UK in any way. These names have not yet been considered by our Banknote Character Advisory Committee. We plan to announce the character for the new £50 banknote in Summer 2019. Aaron Klug Alister Hardy Augustus De Morgan Abraham Bennet Allen Coombs Austin Bradford Hill Abraham Darby Allen McClay Barbara Ansell Abraham Manie Adelstein Alliott Verdon Roe Barbara Clayton Ada Lovelace Alma Howard Barnes Neville Wallis Adam Sedgwick Andrew Crosse Baron Charles Percy Snow Aderlard of Bath Andrew Fielding Huxley Bawa Kartar Singh Adrian Hardy Haworth Angela Hartley Brodie Beatrice "Tilly" Shilling Agnes Arber Angela Helen Clayton Beatrice Tinsley Alan Archibald Campbell‐Swinton Anita Harding Benjamin Gompertz Alan Arnold Griffiths Ann Bishop Benjamin Huntsman Alan Baker Anna Atkins Benjamin Thompson Alan Blumlein Anna Bidder Bernard Katz Alan Carrington Anna Freud Bernard Spilsbury Alan Cottrell Anna MacGillivray Macleod Bertha Swirles Alan Lloyd Hodgkin Anne McLaren Bertram Hopkinson Alan MacMasters Anne Warner -

Belgian Journal of Paediatrics) Verderzijn Krijgen Talrijk: De Leden Ook Gratis Het Tijdschrift “Belgian Journal of Paediatrics”
B P Belgian Journal BELGISCHE VERENIGING of Paediatrics VOOR KINDERGENEESKUNDE J SOCIÉTÉ BELGE DE PÉDIATRIE Publication of the Belgian Society of Paediatrics 2018 - Volume 20 - number 3 - September Theme: Pediatric rheumatology Perspectives : Problem solving approach in pediatric rheumatology Recognition of rheumatic condition at the emergency unit Back pain: when to refer to a paediatric rheumatologist ? A concise review on juvenile spondyloarthritis Beyond recurrent fever, inflammasome-related auto-inflammatory diseases Dermatological presentations of paediatric rheumatic diseases Paediatric non-infectious Uveitis New therapies for children with rheumatic conditions Article An adjusted Bristol Stool Scale for non-toilet-trained children: the Brussels Infant and Toddler Stool Scale (BITSS) Review Article Congenital pulmonary malformations: Case series and review of the literature Case Report Lactose breath tests in pediatrics: “A tailwind towards diagnosis or a whiff of confusion?” Pacemaker implantation as a successful treatment of complicated breath holding spells: a case report and review of the literature Two cases of pyelonephritis following voiding cystourethrography: why this warrants a different approach. Short Communication Seasonality of respiratory syncytial virus (RSV) in Belgium Paediatric Cochrane Corner Antibiotics may be effective to treat prolonged wet cough in children Belgische Vereniging voor Kindergeneeskunde Société Belge de Pédiatrie QUARTERLY VV.U./E.R. S. Cadranel (ULB), M. Raes (KUL) ISSN 2466-8907 (printed version) UZ Leuven, Herestraat 49, 3000 Leuven ISSN 2566-1558 (digital version) E-mail: [email protected] NAAM VAN HET GENEESMIDDEL Enterol 250 mg poeder voor orale suspensie kingen: De bijwerkingen worden hier- Enterol 250 mg harde capsules. Saccharomyces boulardii CNCM I-745. KWALITATIE- onder geklasseerd per orgaansys- VE EN KWANTITATIEVE SAMENSTELLING Enterol 250 mg poeder voor orale suspensie: teem en volgens de frequentie. -

The History of Pediatric Rheumatology
0031-3998/05/5805-0997 PEDIATRIC RESEARCH Vol. 58, No. 5, 2005 Copyright © 2005 International Pediatric Research Foundation, Inc. Printed in U.S.A. The History of Pediatric Rheumatology JANE G. SCHALLER International Pediatric Association, Tufts University School of Medicine, Boston, Massachusetts, 02111 ABSTRACT The care and study of children with rheumatic diseases began biomedical research are adding to our basic understanding of the slowly in the 19th century, with the most attention centered on disease process involved. Pediatric rheumatology has become a rheumatic fever. Other rheumatic diseases of children received well-organized, although underpopulated, specialty that en- little attention until the 1940s. Rheumatic diseases taken together hances recognition and care of affected children and contributes remain a significant cause of chronic illness in children through- to basic research knowledge in infectious disease, immunology, out the world. A number of other conditions that masquerade as and genetics. This review focuses most prominently on the early rheumatic diseases in children also demand recognition and history of pediatric rheumatology and its development as a management. Although ultimate causes and cures of childhood specialty. The recent burgeoning of new biomedical science and rheumatic diseases remain elusive, advances in therapy have new means of treatment will be better told in the historical improved the outlook for affected children, and advances in perspective of years to come. (Pediatr Res 58: 997–1007, 2005) Pediatric rheumatology is one of the newest and least pop- Pediatric rheumatology is closely linked to the field of ulated of the pediatric subspecialties, having come slowly into immunology, and the rheumatic diseases often referred to being only after the Second World War. -

Scottish Paediatric Society and British Paediatric Association
Arch Dis Child: first published as 10.1136/adc.57.10.795 on 1 October 1982. Downloaded from Archives of Disease in Childhood, 1982, 57, 795-809 Scottish Paediatric Society and British Paediatric Association Proceedings of a Joint Meeting A joint meeting between the Scottish Paediatric (Birmingham), C A Campbell (London), N D Carter Society and the British Paediatric Association was (London), K C Chin (Birmingham), S Chong held at Aviemore from 20 to 24 April 1982. 321 (London), G C Close (Manchester), Gaynor F Cole members and 10 associate members attended, (London), C Costalos (Greece), Kathleen L Costeloe together with 3 Heinz Fellows and 216 guests. The (Slough), Marion Crouchman (London), R E Windermere Lecture was given by Professor Mary Cudmore (Liverpool), Jean S Davies (Southampton), Ellen Avery, Thomas Morgan Rotch professor of H Dellagrammaticas (Greece), N R Dennis paediatrics(Harvard Medical School, Boston, Massa- (Southampton), B Donaldson (Glasgow), Margaret chusetts) and the Scottish Paediatric Society Fifth Drummond (Glasgow), Gillian C L Du Mont Jubilee Lecture was given by Professor E M McGirr (London), N V Freeman (Southampton), W J Fysh (Chairman, Scottish Council for Postgraduate (London), Margaret Gallagher (Dublin), Ann Gath Medical Education). (Bury St Edmunds), Jean Golding (Oxford), M A Hall (Southampton), Susanna M Hart (London), A plenary session on Paediatric Urology was D P Heaf (London), J A Hulse (London), R Hume mounted by the British Association of Paediatric (Edinburgh), Mary Johnson (Manchester), P M Surgeons on 23 April. Jones (Newcastle), Rosamond A K Jones (Norwich), copyright. C J H Kelnar (London), Patricia A Kenny (London), The Annual General Meeting of the British Alison M Kerr (Glasgow), S D Levin (London), Paediatric Association was held on Thursday 22 A Lucas (Cambridge), J McKiernan (Cork), A April 1982. -

Abraham Shore Memorial Lectureship by Ronald M
NORTHERN (HIGH)LIGHTS Abraham Shore Memorial Lectureship By Ronald M. Laxer, MDCM, FRCPC his year marks the 20 th anniversary of the Abraham Shore Memorial Lecture. This lec - T ture was established by the friends and family of the late Dr. Abraham Shore, to ensure that Abe’s name remains associated with excellence in pediatric rheumatology. Abe was born in Germany in 1946, and his family immigrated to Vineland, New Jersey, in 1950. Abe grad - uated from the University of Pennsylvania School of Medicine in 1972. He came to Toronto to do a residen - cy in pediatrics, which was followed by a fellowship in pediatric immunology. Abe then went to spend a year with Dr. Barbara Ansell, pioneer in pediatric rheuma - tology, in the United Kingdom; he then spent a year in the division of adult rheumatology at the University of Toronto. Abe was the first pediatrician to be certified in rheumatology by the Royal College of Physicians and Surgeons of Canada. He quickly established a strong reputation, both clinically and as a basic scientist, with ongoing peer-reviewed funding from the Medical Research Council of Canada (now the Canadian Institutes of Health Research [CIHR]), The National Cancer Institute (NCI), and The Arthritis Society (TAS). In addition to his outstanding contributions to list of the lecturers, it is obvious that the goals of the clinical care and basic research, Abe was recognized as lectureship are being met. a skilled educator. Despite suffering from a chronic disease all his life and struggling to establish the specialty of pediatric rheumatology in the country, Abe never complained.