Spring 2017 02 Letter from the President
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

State of New York 2 0 1 8 Villagetaxroll Page 1 County
STATE OF NEW YORK 2 0 1 8 V I L L A G E T A X R O L L PAGE 1 COUNTY - Chautauqua T A X A B L E SECTION OF THE ROLL - 1 VALUATION DATE-JUL 01, 2016 TOWN - Pomfret TAX MAP NUMBER SEQUENCE TAXABLE STATUS DATE-MAR 01, 2017 VILLAGE - Fredonia UNIFORM PERCENT OF VALUE IS 018.25 SWIS - 065801 TAX MAP PARCEL NUMBER PROPERTY LOCATION & CLASS ASSESSMENT EXEMPTION CODE------------------VILLAGE------ ------------ CURRENT OWNERS NAME SCHOOL DISTRICT LAND TAX DESCRIPTION TAXABLE VALUE CURRENT OWNERS ADDRESS PARCEL SIZE/GRID COORD TOTAL SPECIAL DISTRICTS TAX AMOUNT ******************************************************************************************************* 96.03-1-1 ****************** Temple St BILL 1 96.03-1-1 311 Res vac land AG DIST 41720 50 Joy Russell C Jr Fredonia 065801 50 Village Tax 0.00 0.00 Joy Barbara B 101-2-4 50 556 Temple St ACRES 0.05 Fredonia, NY 14063 EAST-0940265 NRTH-0896077 DEED BOOK 2597 PG-120 MAY BE SUBJECT TO PAYMENT FULL MARKET VALUE 274 UNDER AGDIST LAW TIL 2021 TOTAL TAX --- 0.00** ******************************************************************************************************* 96.03-1-3 ****************** 560 Temple St BILL 2 96.03-1-3 210 1 Family Res Village Tax 21,640 761.51 DeChard Jason T Fredonia 065801 3,330 560 Temple St 2015 R & C w/24.2 21,640 Fredonia, NY 14063 101-2-5 FRNT 95.00 DPTH 165.00 EAST-0940286 NRTH-0895992 DEED BOOK 2014 PG-4076 FULL MARKET VALUE 118,575 TOTAL TAX --- 761.51** DATE #1 07/02/18 AMT DUE 761.51 ******************************************************************************************************* -
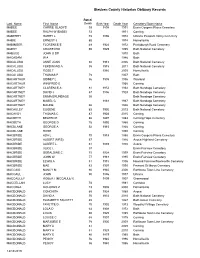
Steuben County Historian Obituary Records
Steuben County Historian Obituary Records Age at Last_Name First_Name Death Birth Year Death Year Cemetery/Town Name MABEE CARRIE GLADYS 93 1898 1991 Erwin Coopers Plains Cemetery MABEE RALPH W (BABE) 73 1971 Corning MABERRY HARRY L 78 1896 1974 Urbana Pleasant Valley Cemetery MABIE ERNEST J 80 1974 Hornellsville MABINGER FLORENCE E 49 1923 1972 Prattsburgh Rural Cemetery MABRY CRAWFORD 66 1929 1995 Bath National Cemetery MABUCE JOHN O DR 1970 Bath MACADAM W A 1946 Bath MACALUSO ANNE JOAN 92 1913 2006 Bath National Cemetery MACALUSO FERDINAND A 95 1915 2011 Bath National Cemetery MACALUSO ROSE T 1916 2005 Hornellsville MACALUSO THOMAS P 78 2007 Bath MACARTHUR SIDNEY L 86 1909 1996 Wayland MACARTHUR WINIFRED E 1968 Corning MACARTNEY CLARENCE A 81 1852 1934 Bath Nondaga Cemetery MACARTNEY DAVID J 67 1856 1923 Bath Nondaga Cemetery MACARTNEY EMMA BRUNDAGE 50 Bath Nondaga Cemetery MACARTNEY MABEL C 1861 1947 Bath Nondaga Cemetery MACARTNEY MAUDE 66 1946 Bath Nondaga Cemetery MACAULEY EUGENE 83 1930 2013 Bath National Cemetery MACAVOY THOMAS C 87 1928 2015 Corning MACBETH BEATRICE 86 1897 1983 Corning Hope Cemetery MACBETH GEORGE D 76 1892 1968 Corning MACBLANE GEORGE A 52 1915 1968 Corning MACBLANE ROSE 1990 Corning MACBRIDE ADA L 70 1918 1988 Erwin Coopers Plains Cemetery MACBRIDE ALBERT (MRS) 57 1946 Avoca Highland Cemetery MACBRIDE ALBERT L 61 1889 1950 Avoca MACBRIDE ALICE I. 97 Erwin Fairview Cemetery MACBRIDE GERALDINE C 75 1924 1999 Erwin Fairview Cemetery MACBRIDE JOHN W 77 1918 1995 Erwin Fairview Cemetery MACBRIDE LEWIS A 81 1915 -

SCRIPPS DISCOVERS FALL 2010 Selective Inhibition of BMK1 Suppresses Tumor Growth
SC RIPPS DISC OVERS Accelerating Discoveries, Saving Lives A Newsletter for Philanthropists Published Quarterly by The Scripps Research Institute WINTER 2010 | VOL 7 | NO 1 California-Florida RESEARCH UPDATE Scripps Research Scientists Develop Molecular Test Providing a New Pathway for Identifying Obesity and Diabetes Drugs cientists at The Scripps Research Institute The Worm Institute for Research and Medicine have designed a new molecular test that at Scripps Research. Swill allow researchers to look for potential Janda and Amanda Garner, Ph.D., a research drugs targeting a human metabolic enzyme believed associate in his laboratory, described the new to stimulate the appetite and play a role in diabetes. approach—which may also prove useful for The new test, which the scientists call a simple investigating other enzymes involved in a assay, will allow researchers to look through variety of diseases—in an advance, online Early hundreds of thousands of compounds for those that Edition of the journal Angewandte Chemie on have potential to block the action of an enzyme September 15, 2010. known as ghrelin O-acyltransferase (GOAT). If Even though GOAT was only discovered drugs can be found that safely suppress the action recently, in 2008, scientists had speculated for of GOAT, they may help people who have clinical years that it had to exist. After all, its target (ghrelin, problems with appetite, obesity, and diabetes. or the “G” in the acronym GOAT) had been Professor Kim Janda “There hasn’t been a simple screen until now,” known for more than a decade. says Kim D. Janda, Ph.D., a professor in the Ghrelin, a small peptide hormone that is Departments of Chemistry and Immunology mainly produced in the stomach, signals hunger, and Microbial Science, member of The Skaggs typically before meals. -

Dear Graduates, Nova Southeastern University Takes
Dear Graduates, Nova Southeastern University takes enormous pride in your success. On behalf of NSU’s faculty, staff, and Board of Trustees, I salute your academic and personal achievement. You have reached this milestone through hard work and intellectual effort, and we are pleased to recognize your dedication with today’s commencement ceremony. Reflect on the gifts of knowledge and support you have received. Celebrate the friendships, skills, and strengths you have forged. Embrace the opportunity to apply these treasures as you start a new chapter in your life, hopefully, following your passion, not your fortune. Our best wishes are with you today and in the future. Congratulations! George L. Hanbury II, Ph.D. NSU President and CEO CEREMONY SCHEDULE May 16, 2021, at 9:30 a.m. Shepard Broad College of Law May 17, 2021, at 9:30 a.m. All Undergraduate Degrees May 17, 2021, at 3:00 p.m. H. Wayne Huizenga College of Business and Entrepreneurship May 18, 2021, at 9:30 a.m. College of Dental Medicine Dr. Kiran C. Patel College of Allopathic Medicine Dr. Kiran C. Patel College of Osteopathic Medicine College of Psychology May 18, 2021, at 3:00 p.m. College of Optometry College of Pharmacy Ron and Kathy Assaf College of Nursing May 19, 2021, at 9:30 a.m. Abraham S. Fischler College of Education and School of Criminal Justice Halmos College of Arts and Sciences College of Computing and Engineering May 19, 2021, at 3:00 p.m. Dr. Pallavi Patel College of Health Care Sciences 2 CLASS OF 2021 THE ACADEMIC PROCESSION Grand Marshal Degree Candidates Members of the Faculty Members of the Board of Trustees Distinguished Guests University Officials 3 WELCOME TO THE 2021 COMMENCEMENT CEREMONIES for NOVA SOUTHEASTERN UNIVERSITY May 16 to 19, 2021 4 CLASS OF 2021 ORDER OF EXERCISES SHEPARD BROAD COLLEGE OF LAW MAY 16, 2021, AT 9:30 A.M. -

BRIDGET BELL, Formerly CARROLL, Nee HOPKINS C1833-1915
BRIDGET BELL, formerly CARROLL, nee HOPKINS c1833-1915 TRACING THE LONG JOURNEY OF A YOUNG ORPHAN GIRL FROM COUNTY GALWAY, IRELAND TO BOURKE, NEW SOUTH WALES, AUSTRALIA AUGUST 10, 2018 KAYE SCHOFIELD [email protected] Introduction Bridget Hopkins was one of 230 Irish orphan girls who sailed into Sydney on the ship Digby on 4 April 1849, and one of more than 4,000 orphan girls who came from 118 different Irish workhouses to Sydney, Melbourne and Adelaide under the Earl Grey Famine Orphan Scheme 1848-1850. She was my maternal great-great-grandmother. While the broad arc of her long and eventful life had been documented, information was quite fragmented, details were scant and their accuracy uncertain. This story reflects my efforts to fill in some details and my continuing interest in understanding the historical context of my family’s history. I had also hoped to find Bridget’s own voice, but she has remained silent throughout. This story builds on and incorporates the efforts of many others who have been researching the Hopkins-Carroll-Bell family history for years, well before I started. In particular I would like to acknowledge the research of some of Bridget’s other descendants: the late Pat Willcox whose 2002 article on Bridget first sparked my interest in finding out more; the late Roy Mitchell, Bridget’s great- grandson, who was passionate about his family tree and genealogy and shared that knowledge freely with so many people; Marie T. Cribbin, Beth Atkinson and Karleen Reilly who, like me, are Bridget’s great-great-granddaughters and who have been so generous in sharing their research, photos and stories with me. -

Handbook of Child Psychology and Developmental Science
HANDBOOK OF CHILD PSYCHOLOGY AND DEVELOPMENTAL SCIENCE Seventh Edition Volume 2 Cognitive Processes Volume Editors LYNN S. LIBEN ULRICH MÜLLER Editor-in-Chief RICHARD M. LERNER Cover design: Wiley This book is printed on acid-free paper. Copyright © 2015 by John Wiley & Sons, Inc. All rights reserved. Published by John Wiley & Sons, Inc., Hoboken, New Jersey. Published simultaneously in Canada. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, pho- tocopying, recording, scanning, or otherwise, except as permitted under Section 107 or 108 of the 1976 United States Copyright Act, without either the prior written permission of the Publisher, or authorization through payment of the appropriate per-copy fee to the Copyright Clearance Center, Inc., 222 Rosewood Drive, Danvers, MA 01923, (978) 750-8400, fax (978) 646-8600, or on the web at www.copyright.com. Requests to the Publisher for permission should be addressed to the Permissions Department, John Wiley & Sons, Inc., 111 River Street, Hoboken, NJ 07030, (201) 748-6011, fax (201) 748-6008. Limit of Liability/Disclaimer of Warranty: While the publisher and author have used their best efforts in preparing this book, they make no representations or warranties with respect to the accuracy or completeness of the contents of this book and specifically disclaim any implied warranties of merchantability or fitness for a particular purpose. No warranty may be created or extended by sales representatives or written sales materials. The adviceandstrategies contained herein may not be suitable for your situation. You should consult with a professional where appropriate. -

Dickens' Holiday Classic
Dickens’ Holiday Classic A VIRTUAL TELLING OF A CHRISTMAS CAROL DECEMBER 19–31, 2020 Inside IN PICTURES Behind the Lens • 3 WELCOME From Artistic Director Joseph Haj • 5 GUTHRIE SPOTLIGHT GUTHRIE SPOTLIGHT Welcome to Dickens’ Holiday Classic • 6 To Our First-Time Patrons • 6 DICKENS’ HOLIDAY CLASSIC Cast, Creative, Film Production and Native Artist Fellows • 11 Biographies • 12 PLAY FEATURES E.G. Bailey and Joseph Haj in Conversation • 15 Changing Tunes in Changing Times • 17 Meet the Native Artist Fellows • 20 A Christmas Carol Memories From Patrons • 23 PLAY FEATURE Backstory • 26 From the Adapters/Directors • 15 SUPPORTERS Annual Fund Contributors • 29 Corporate, Foundation and Public Support • 37 WHO WE ARE Board of Directors and Guthrie Staff •38 GOOD TO KNOW Virtual Viewing Guide • 39 PLAY FEATURE Stories From Productions Past • 23 Guthrie Theater Program Volume 58, Issue 1 • Copyright 2020 818 South 2nd Street, Minneapolis, MN 55415 EDITOR Johanna Buch ADMINISTRATION 612.225.6000 GRAPHIC DESIGNER/COVER DESIGN Brian Bressler BOX OFFICE 612.377.2224 or 1.877.447.8243 (toll-free) CONTRIBUTORS E.G. Bailey, Ernest Briggs, Joseph Haj, guthrietheater.org • Joseph Haj, Artistic Director Margaret Leigh Inners, Katie “KJ” Johns, Tom Mays, Sam Aros Mitchell, Carla Steen. Special thanks to Guthrie The Guthrie creates transformative theater experiences that ignite the patrons for sharing their A Christmas Carol memories. imagination, stir the heart, open the mind and build community through the illumination of our common humanity. The Guthrie program is published by the Guthrie Theater. 2 \ GUTHRIE THEATER • DICKENS’ HOLIDAY CLASSIC IN PICTURES Behind the Lens Two artistic worlds collided for the making of Dickens’ Holiday Classic: theater and film. -

Breakthroughs of 2004 Endeavor VOLUME SEVEN NUMBER THREE Winter 2004/05
VOLUME SEVEN NUMBER THREE Endeavor Breakthroughs of 2004 Endeavor VOLUME SEVEN NUMBER THREE Winter 2004/05 This issue of Endeavor magazine features breakthroughs of 2004 at The Scripps Research Institute. Among many significant scientific milestones this year: the development of potential treatments for certain kinds of blindness that currently have no cure, significant findings on the molecular roots of alcoholism, innovative therapeu- tic strategies for heart attack and stroke, and a new hypothesis on the causes of Alzheimer’s and Parkinson’s diseases. featured page Research Offers New Hope in the Search to Treat Blindness: 6 Martin Friedlander Explores the Potential of Stem Cell Therapy for Restoring Vision Working to Solve the Alzheimer’s and Parkinson’s Puzzles: 10 Jeffery Kelly Proposes a New Explanation for Neurological Disorders Value Added: 14 David Cheresh Conducts Basic Research with Therapeutic Potential At the Roots of Alcoholism: 18 Cindy Ehlers Finds Molecular Answers to an Urgent Social Problem also President’s Introduction 1 Focus on Florida 22 Interview with Harry Orf 24 Financial Highlights 26 Letter from the Development Committee Chair 27 Development Report 29 ENDEAVOR IS A PUBLICATION OF THE SCRIPPS RESEARCH INSTITUTE Year in Review 2004 PRESIDENT’S INTRODUCTION Leadership is about many things: a passionate commitment to pursuing your goals, a vision for the future and an adherence to staying the course, a record of solid performance and achievement, a spirit of innovation and creativity. As an organization, I believe that The Scripps Research Institute has firmly established its leadership position in the international scientific community by remaining true to these basic tenets, by continuing to promulgate a record of outstanding scientific achievements, by attracting extraordinarily talented scientific faculty, staff, students, and board members, and by extending the reach of its capabilities by developing our major new initiative in Florida. -

BSU 2015-2016 Annual Report (Pdf)
Contents 1 A MESSAGE FROM 16 STUDENT ACHIEVEMENTS THE PRESIDENT STUDENT WINS SCHOLARSHIP, SERVES ABROAD 2 ACADEMICS & RESEARCH 18 ATHLETICS RISE OF UNDERGRADUATE RESEARCH BULLDOGS FOOTBALL THE VIEW FROM ABROAD MAKES A HISTORIC RUN NEW EDUCATION PROGRAMS BOWLING TEAM WINS SECOND CONSECUTIVE, MEET CRITICAL NEED SIXTH OVERALL CHAMPIONSHIP BREAKING NEW GROUND 22 PHILANTHROPY IN ACADEMICS YOUR GIFT MAKES A DIFFERENCE 8 STUDENT SUCCESS TOM JOYNER FOUNDATION A FORMULA FOR FRESHMAN SUCCESS SUPPORTS BSU SCHOLARSHIPS 10 FACULTY RECOGNITION 26 DONORS PROFESSORS’ AWARDS 40 FINANCIALS ADVANCE ACADEMIC PROGRAMS 42 ADMINISTRATION 12 150TH ANNIVERSARY WRAP-UP 150TH CELEBRATION CLOSES WITH HISTORIC MARKER 14 STUDENT LIFE MALE INITIATIVE HELPS LOCAL YOUTH II | BOWIE STATE UNIVERSITY ANNUAL REPORT 2015–2016 A MESSAGE FROM THE PRESIDENT A Message from the President hen I became president of Bowie State University students also earned prestigious honors as they participated Win September 2006, I had a vision to transform the in research conferences and various competitions. university into the nation’s best public comprehensive Our alumni and donors are making a difference in the university. I find it very gratifying to know that Bowie lives of students who receive scholarships to keep them State University has made significant progress toward in school. Many supporters took the added step of that goal and is now widely regarded as one of the very contributing to the yearlong Tom Joyner Foundation best higher educational values in America. Hence, the “School of the Month” campaign, raising an additional university is well positioned for continued greatness. $60,000 toward student scholarships. Examining our advancements in academics and research over the past year provides a framework for viewing our Thanks to the dedicated faculty, staff, students, alumni success. -

Last Name First Name Voters ID # Date the City Received the Ballot
Date the City Received the Last Name First Name Voters ID # Ballot By Mail Naumann Brent D. 1204609320 3/28/2020 Naumann David R. 1204609312 3/28/2020 Bruns Florine M 1029504433 3/28/2020 Gillespie James D. 1029519520 3/28/2020 Naumann Beverly M. 1204609331 3/30/2020 Rowell Billie Doralene 1029628664 3/30/2020 Troell Charles E. 1029544038 3/30/2020 Wahl Deborah L. 1029548233 3/30/2020 Troell Dorothy 1029447034 3/30/2020 Plantz John H. 1029547825 3/30/2020 Hirtzel Lawrence E. 2139626217 3/30/2020 Munson Margaret A. 1029644735 3/30/2020 Plantz Margaret E. 1029516136 3/30/2020 Lange Michelle D. 1029512156 3/30/2020 Floyd Peggy Crocker 1029507644 3/30/2020 Wahl Russell Monroe 1029548222 3/30/2020 Forsha Jeri Ann 1205876016 3/30/2020 Geist Larry Allen 1206119197 3/30/2020 Rees Marjorie J. 1029547962 3/30/2020 Heard Norma J. 1034026225 3/30/2020 Heard Walter Thomas 1034026239 3/30/2020 Lewis Judy C. 1156391452 3/30/2020 Gillespie Alene D. 1029512847 3/30/2020 Spousta Allen F. 1029620050 3/30/2020 Moellering Alton M. 1029429156 3/30/2020 Westfall Connie L. 2154944407 3/30/2020 Moellering Dorothy L. Klaemer 1029530497 3/30/2020 Grunau Ernestine Zitzmann 1029600240 3/30/2020 Grunau Kurt 1029521321 3/30/2020 Greding Marcia J. 1059920887 3/30/2020 Davis Martha A. 1029555713 3/30/2020 Spousta Patricia E. 1029620066 3/30/2020 Westfall Robert S. 2158985648 3/30/2020 Westfall Scott A. 2158983931 3/30/2020 Stone Annie M. 1207457826 3/31/2020 Pankov Karen S. -

Download The
ii Science as a Superpower: MY LIFELONG FIGHT AGAINST DISEASE AND THE HEROES WHO MADE IT POSSIBLE By William A. Haseltine, PhD YOUNG READERS EDITION iii Copyright © 2021 by William A. Haseltine, PhD All rights reserved. No part of this book may be used or reproduced by any means, graphic, electronic, or mechanical, including photocopying, recording, taping, or by any information storage retrieval system, without the written permission of the publisher except in the case of brief quotations embodied in critical articles and reviews. iv “If I may offer advice to the young laboratory worker, it would be this: never neglect an extraordinary appearance or happening.” ─ Alexander Fleming v CONTENTS Introduction: Science as A Superpower! ............................1 Chapter 1: Penicillin, Polio, And Microbes ......................10 Chapter 2: Parallax Vision and Seeing the World ..........21 Chapter 3: Masters, Mars, And Lasers .............................37 Chapter 4: Activism, Genes, And Late-Night Labs ........58 Chapter 5: More Genes, Jims, And Johns .........................92 Chapter 6: Jobs, Riddles, And Making A (Big) Difference ......................................................................107 Chapter 7: Fighting Aids and Aiding the Fight ............133 Chapter 8: Down to Business ...........................................175 Chapter 9: Health for All, Far and Near.........................208 Chapter 10: The Golden Key ............................................235 Glossary of Terms ..............................................................246 -

Supreme Court of the United States
No. 17-290 IN THE Supreme Court of the United States MERCK SHARP & DOHME CORP., Petitioner, v. DORIS ALBRECHT, ET AL., Respondents. On Writ of Certiorari To The United States Court of Appeals For The Third Circuit BRIEF FOR PETITIONER STEPHANIE PARKER SHAY DVORETZKY JONES DAY Counsel of Record 1420 Peachtree St., NE YAAKOV M. ROTH Suite 800 JEFFREY R. JOHNSON Atlanta, GA 30309 JONES DAY 51 Louisiana Ave., NW BENJAMIN M. FLOWERS Washington, DC 20001 JONES DAY (202) 879-3939 325 John H. McConnell Blvd. [email protected] Suite 600 Columbus, OH 43215 Counsel for Petitioner i QUESTION PRESENTED In Wyeth v. Levine, 555 U.S. 555 (2009), this Court held that the FDA’s approval of a drug label does not, standing alone, insulate the manufacturer from failure-to-warn liability under state tort law. At the same time, the Court recognized that if “the FDA would not have approved” the label demanded by state law, then the manufacturer could invoke an “impossibility” preemption defense. Id. at 571. In this case, it was “undisputed” that (i) “the FDA was aware of the possible link” between petitioner’s drug and the risk at issue; (ii) petitioner “submitted a comprehensive safety update to the FDA reporting … numerous studies” finding “such an association”; (iii) petitioner “proposed warning language” about this risk, but the FDA “rejected” it; (iv) the FDA stated that the “conflicting nature of the literature d[id] not provide a clear path forward” and that it needed “more time” to consider “the issue of a precaution”; and (v) only later, after a report from a task force, did the FDA become “confident” that an association “potentially” existed.