Antilisterial Effect of a Natural Formulation Based on Citrus Extract in Ready-To-Eat Foods
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Full Circle Magazine #33 Contents ^ Full Circle Ubuntu Women P.28
full circle ISSUE #33 - January 2010 CCRREEAATTEE AA MMEEDDIIAA CCEENNTTEERR WWIITTHH UUBBUUNNTTUU,, AANN AACCEERR RREEVVOO && BBOOXXEEEE full circle magazine #33 contents ^ full circle Ubuntu Women p.28 Program In Python - Pt7 p.08 Ubuntu Games p.31 My Story p.19 MOTU Interview p.24 Read how Ubuntu is used in public education, and why one man made the switch to Linux. Ubuntu, Revo & Boxee p.13 Command & Conquer p.05 The Perfect Server - Pt3 p.15 Review - Exaile p.23 Letters p.26 Top 5 - Sync. Clients p.35 The articles contained in this magazine are released under the Creative Commons Attribution-Share Alike 3.0 Unported license. This means you can adapt, copy, distribute and transmit the articles but only under the following conditions: You must attribute the work to the original author in some way (at least a name, email or URL) and to this magazine by name ('full circle magazine') and the URL www.fullcirclemagazine.org (but not attribute the article(s) in any way that suggests that they endorse you or your use of the work). If you alter, transform, or build upon this work, you must distribute the resulting work under the same, similar or a compatible license. full circle magazine #33 contents ^ EDITORIAL This magazine was created using : Welcome to another issue of Full Circle magazine. ast month, Andrew gave us his Top 5 Media Center applications. This month I've written a How-To on using Ubuntu on an Acer Aspire Revo to create the foundation for Boxee. For under £150 I've created a fantastic media center L which not only looks great, it's fully customizable! That's my media center story, but don't forget to read the My Story articles which this month focus on Ubuntu, Linux and open-source in public education, as well as how one man went from using old (modern at the time) computers, to using Ubuntu. -

The Gnome Desktop Comes to Hp-Ux
GNOME on HP-UX Stormy Peters Hewlett-Packard Company 970-898-7277 [email protected] THE GNOME DESKTOP COMES TO HP-UX by Stormy Peters, Jim Leth, and Aaron Weber At the Linux World Expo in San Jose last August, a consortium of companies, including Hewlett-Packard, inaugurated the GNOME Foundation to further the goals of the GNOME project. An organization of open-source software developers, the GNOME project is the major force behind the GNOME desktop: a powerful, open-source desktop environment with an intuitive user interface, a component-based architecture, and an outstanding set of applications for both developers and users. The GNOME Foundation will provide resources to coordinate releases, determine future project directions, and promote GNOME through communication and press releases. At the same conference in San Jose, Hewlett-Packard also announced that GNOME would become the default HP-UX desktop environment. This will enhance the user experience on HP-UX, providing a full feature set and access to new applications, and also will allow commonality of desktops across different vendors' implementations of UNIX and Linux. HP will provide transition tools for migrating users from CDE to GNOME, and support for GNOME will be available from HP. Those users who wish to remain with CDE will continue to be supported. Hewlett-Packard, working with Ximian, Inc. (formerly known as Helix Code), will be providing the GNOME desktop on HP-UX. Ximian is an open-source desktop company that currently employs many of the original and current developers of GNOME, including Miguel de Icaza. They have developed and contributed applications such as Evolution and Red Carpet to GNOME. -

US EPA, Pesticide Product Label, Crew,10/08/2019
U.S. ENVIRONMENTAL PROTECTION AGENCY EPA Reg. Number: Date of Issuance: Office of Pesticide Programs Registration Division (7505P) 62719-742 10/8/19 1200 Pennsylvania Ave., N.W. Washington, D.C. 20460 NOTICE OF PESTICIDE: Term of Issuance: X Registration Reregistration Conditional (under FIFRA, as amended) Name of Pesticide Product: Crew Name and Address of Registrant (include ZIP Code): Jennifer Hughes Dow AgroSciences LLC 9330 Zionsville Rd 308/2E Indianapolis, IN 46268-1054 Note: Changes in labeling differing in substance from that accepted in connection with this registration must be submitted to and accepted by the Registration Division prior to use of the label in commerce. In any correspondence on this product always refer to the above EPA registration number. On the basis of information furnished by the registrant, the above named pesticide is hereby registered under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA). Registration is in no way to be construed as an endorsement or recommendation of this product by the Agency. In order to protect health and the environment, the Administrator, on his motion, may at any time suspend or cancel the registration of a pesticide in accordance with the Act. The acceptance of any name in connection with the registration of a product under this Act is not to be construed as giving the registrant a right to exclusive use of the name or to its use if it has been covered by others. This product is conditionally registered in accordance with FIFRA section 3(c)(7)(A). You must comply with the following conditions: 1. -

Biological Applications of Surfaces with Extreme Wettabilities by Sarah
Biological Applications of Surfaces with Extreme Wettabilities by Sarah A. Snyder A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Materials Science and Engineering) in the University of Michigan 2019 Doctoral Committee: Assistant Professor Geeta Mehta, Co-Chair Assistant Professor Anish Tuteja, Co-Chair Associate Professor Sunitha Nagrath Assistant Professor J. Scott VanEpps Sarah A. Snyder [email protected] ORCID iD: 0000-0002-6741-297X © Sarah A. Snyder 2019 Dedication To my parents ii Acknowledgements First and foremost, I need to thank Prof. Geeta Mehta and Prof. Anish Tuteja. As co- advisors you continually encouraged me to be the best that I can be both academically and personally. I cannot thank you two enough, you not only taught me to push the boundaries with my ideas but also gave me the tools to make those ideas realities. Thanks also to my committee members, Prof. Sunitha Nagrath and Prof. Scott VanEpps. Scott, thank you so much for the insight and encouragement you provided. I have truly enjoyed working with you and your lab. I want to thank the National Science Foundation’s Graduate Research Fellowship Program for their resources and support during my time at the University of Michigan. To the lab members of both the PSI and ECM groups, thank you. Thank you for the encouragement, thank you for teaching me valuable skills, and thank you for just generally helping me get through the day. Matt, thank you, I could not have done this without all of your help and support. Catherine and Caymen, thank you for always making me smile, you two made lab fun. -

Scientific Program Table of Contents
Scientific Program Table of Contents Sunday, July 11 SYMPOSIA AND ORAL SESSIONS SYMPOSIUM: Triennial Growth Symposium: Dietary Regulation of Growth and Development . 53 ASAS Western Section Graduate Paper Competition. 53 SYMPOSIUM: National Extension Workshop: The Impact of Major Food Policy Shifts on the US Food Supply and its Producers: Animal Welfare Issues . 55 SYMPOSIUM: Informal Nutrition Symposium: Connecting Nutrition, Biochemistry, Genetics, Physiology, and Microbiology to Enhance Our Knowledge in Improving Animal Agriculture . 56 Johne’s Disease Integrated Program (JDIP) Meeting . 56 Late Breaking/Original Research . 56 Monday, July 12 POSTER PRESENTATIONS Animal Behavior and Well-Being: Livestock. 57 Animal Health: Infl ammation, Infection, and Stress . 58 Animal Health-Johne’s Disease (JDIP): Johne’s Disease . 60 Breeding and Genetics: Beef Cattle. 61 Food Safety 1 . 63 Forages and Pastures: Dairy Forages and Forage Quality . 63 Forages and Pastures: Grazing and Forage Management . 65 Immunology and Pathology: Poultry Immunology and Pathology . 66 Lactation Biology 1. 66 Meat Science and Muscle Biology: Beef Quality . 67 Scientific Graduate Student Poster Competition: National ADSA Dairy Foods Poster. 69 TOC Graduate Student Poster Competition: National ADSA Production MS Poster. 70 Graduate Student Poster Competition: National ADSA Production PhD Poster . 70 Nonruminant Nutrition: Amino Acids . 71 Nonruminant Nutrition: Feed Ingredients . 73 Physiology and Endocrinology: Nutritional Effects on Reproduction and Development . 75 Physiology and Endocrinology: Pregnancy. 76 Physiology and Endocrinology: Reproductive Endocrinology . 76 Physiology and Endocrinology: Reproductive Management. 77 Production, Management and the Environment: Microbiology . 77 Production, Management and the Environment: Poultry . 77 Production, Management and the Environment: Small Ruminant . 79 Production, Management and the Environment: Swine . -

Personal Knowledge Models with Semantic Technologies
Max Völkel Personal Knowledge Models with Semantic Technologies Personal Knowledge Models with Semantic Technologies Max Völkel 2 Bibliografische Information Detaillierte bibliografische Daten sind im Internet über http://pkm. xam.de abrufbar. Covergestaltung: Stefanie Miller Herstellung und Verlag: Books on Demand GmbH, Norderstedt c 2010 Max Völkel, Ritterstr. 6, 76133 Karlsruhe This work is licensed under the Creative Commons Attribution- ShareAlike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-sa/3.0/ or send a letter to Creative Commons, 171 Second Street, Suite 300, San Fran- cisco, California, 94105, USA. Zur Erlangung des akademischen Grades eines Doktors der Wirtschaftswis- senschaften (Dr. rer. pol.) von der Fakultät für Wirtschaftswissenschaften des Karlsruher Instituts für Technologie (KIT) genehmigte Dissertation von Dipl.-Inform. Max Völkel. Tag der mündlichen Prüfung: 14. Juli 2010 Referent: Prof. Dr. Rudi Studer Koreferent: Prof. Dr. Klaus Tochtermann Prüfer: Prof. Dr. Gerhard Satzger Vorsitzende der Prüfungskommission: Prof. Dr. Christine Harbring Abstract Following the ideas of Vannevar Bush (1945) and Douglas Engelbart (1963), this thesis explores how computers can help humans to be more intelligent. More precisely, the idea is to reduce limitations of cognitive processes with the help of knowledge cues, which are external reminders about previously experienced internal knowledge. A knowledge cue is any kind of symbol, pattern or artefact, created with the intent to be used by its creator, to re- evoke a previously experienced mental state, when used. The main processes in creating, managing and using knowledge cues are analysed. Based on the resulting knowledge cue life-cycle, an economic analysis of costs and benefits in Personal Knowledge Management (PKM) processes is performed. -

Screenshot Showcase Texstar
Volume 107 December, 2015 HHaappppyy HHoolliiddaayyss 2015 Holiday Gift Guide: What Programming Meemaw's Picks Language? 2015 Holiday Gift Guide: The Chimpbox: YouCanToo's Picks A Review 2015 Holiday Gift Guide: Game Zone: Stick 'Em parnote's Picks Up 2: Paper Adventures PCLinuxOS Family Member Inkscape Tutorial: Another Spotlight: bkstan Fun Text Effect Inkscape Tutorial: Creating Tip Top Tips: Running A Jigsaw Puzzle Using The Multiple Logitech Devices Lasercut Extension And more inside ... From One USB Receiver PCLinuxOS Magazine Page 1 Table Of Contents 3 Welcome From The Chief Editor 4 Inkscape Tutorial: Another Fun Text Effect The PCLinuxOS name, logo and colors are the trademark of 5 Screenshot Showcase Texstar. 6 The ChimpBox: A Review The PCLinuxOS Magazine is a monthly online publication containing PCLinuxOS-related materials. It is published 7 Screenshot Showcase primarily for members of the PCLinuxOS community. The magazine staff is comprised of volunteers from the 8 ms_meme's Nook: No Place Like The Forum PCLinuxOS community. 9 PCLinuxOS Recipe Corner: Tofu Parmigiana Visit us online at http://www.pclosmag.com 10 2015 Holiday Gift Guide: parnote's Picks This release was made possible by the following volunteers: 13 2015 Holiday Gift Guide: Meemaw's Picks Chief Editor: Paul Arnote (parnote) Assistant Editor: Meemaw 15 Screenshot Showcase Artwork: Sproggy, Timeth, ms_meme, Meemaw Magazine Layout: Paul Arnote, Meemaw, ms_meme 16 2015 Holiday Gift Guide: YouCanToo's Picks HTML Layout: YouCanToo 19 PCLinuxOS Friends & Family Member -

Upgrade Issues
Upgrade issues Graph of new conflicts libsiloh5-0 libhdf5-lam-1.8.4 (x 3) xul-ext-dispmua (x 2) liboss4-salsa-asound2 (x 2) why sysklogd console-cyrillic (x 9) libxqilla-dev libxerces-c2-dev iceape xul-ext-adblock-plus gnat-4.4 pcscada-dbg Explanations of conflicts pcscada-dbg libpcscada2-dev gnat-4.6 gnat-4.4 Similar to gnat-4.4: libpolyorb1-dev libapq-postgresql1-dev adacontrol libxmlada3.2-dev libapq1-dev libaws-bin libtexttools2-dev libpolyorb-dbg libnarval1-dev libgnat-4.4-dbg libapq-dbg libncursesada1-dev libtemplates-parser11.5-dev asis-programs libgnadeodbc1-dev libalog-base-dbg liblog4ada1-dev libgnomeada2.14.2-dbg libgnomeada2.14.2-dev adabrowse libgnadecommon1-dev libgnatvsn4.4-dbg libgnatvsn4.4-dev libflorist2009-dev libopentoken2-dev libgnadesqlite3-1-dev libnarval-dbg libalog1-full-dev adacgi0 libalog0.3-base libasis2008-dbg libxmlezout1-dev libasis2008-dev libgnatvsn-dev libalog0.3-full libaws2.7-dev libgmpada2-dev libgtkada2.14.2-dbg libgtkada2.14.2-dev libasis2008 ghdl libgnatprj-dev gnat libgnatprj4.4-dbg libgnatprj4.4-dev libaunit1-dev libadasockets3-dev libalog1-base-dev libapq-postgresql-dbg libalog-full-dbg Weight: 5 Problematic packages: pcscada-dbg hostapd initscripts sysklogd Weight: 993 Problematic packages: hostapd | initscripts initscripts sysklogd Similar to initscripts: conglomerate libnet-akamai-perl erlang-base screenlets xlbiff plasma-widget-yawp-dbg fso-config- general gforge-mta-courier libnet-jifty-perl bind9 libplack-middleware-session-perl libmail-listdetector-perl masqmail libcomedi0 taxbird ukopp -

* His Is the Original Ubuntuguide. You Are Free to Copy This Guide but Not to Sell It Or Any Derivative of It. Copyright Of
* his is the original Ubuntuguide. You are free to copy this guide but not to sell it or any derivative of it. Copyright of the names Ubuntuguide and Ubuntu Guide reside solely with this site. This guide is neither sold nor distributed in any other medium. Beware of copies that are for sale or are similarly named; they are neither endorsed nor sanctioned by this guide. Ubuntuguide is not associated with Canonical Ltd nor with any commercial enterprise. * Ubuntu allows a user to accomplish tasks from either a menu-driven Graphical User Interface (GUI) or from a text-based command-line interface (CLI). In Ubuntu, the command-line-interface terminal is called Terminal, which is started: Applications -> Accessories -> Terminal. Text inside the grey dotted box like this should be put into the command-line Terminal. * Many changes to the operating system can only be done by a User with Administrative privileges. 'sudo' elevates a User's privileges to the Administrator level temporarily (i.e. when installing programs or making changes to the system). Example: sudo bash * 'gksudo' should be used instead of 'sudo' when opening a Graphical Application through the "Run Command" dialog box. Example: gksudo gedit /etc/apt/sources.list * "man" command can be used to find help manual for a command. For example, "man sudo" will display the manual page for the "sudo" command: man sudo * While "apt-get" and "aptitude" are fast ways of installing programs/packages, you can also use the Synaptic Package Manager, a GUI method for installing programs/packages. Most (but not all) programs/packages available with apt-get install will also be available from the Synaptic Package Manager. -
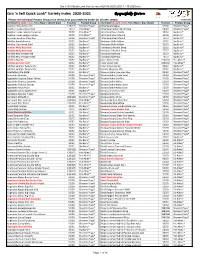
Gro 'N Sell Quick Look* Variety Index 2020-2021 Respectfully Gro'n ™
Gro 'n Sell Quick Look Variety Index ALPHA 2020-2021 11.30.2020.xlsx Gro 'n Sell Quick Look* Variety Index 2020-2021 Respectfully Gro'n ™ *Please see individual Product Group price sheets from your preferred broker for all other details. 11.30.2020 Variety (Red, bold = new; Terra Nova = shaded blue) Tray Size Product Group Variety (Red, bold = new; Terra Nova = blue shade) Tray Size Product Group Abutilon Bella Select Mix 128/125 Bloomin Plugs® Artemisia Makana 51/50 Bloomin Plugs® Abutilon Lucky Lantern Red 72/72 Pot-Ables™ Artemisia Parfum de Ethiopia 51/50 Bloomin Plugs® Abutilon Lucky Lantern Tangerine 72/72 Pot-Ables™ Artemisia Powis Castle 32/32 Big Burly® Abutilon Lucky Lantern Yellow 72/72 Pot-Ables™ Artemisia Silver Mound 32/32 Big Burly® Acalypha pendula (Chenille) 51/50 Bloomin Plugs® Artemisia Silver Mound 72/72 Big Burly® Achillea Appleblossom 32/32 Big Burly® Asclepias Hello Yellow 32/32 Big Burly® Achillea Coronation Gold 32/32 Big Burly® Asclepias Hello Yellow 72/72 Big Burly® Achillea Milly Rock Red 32/32 Big Burly® Asclepias Silky Red Deep 32/32 Big Burly® Achillea Milly Rock Rose 32/32 Big Burly® Asclepias Silky Red Deep 72/72 Big Burly® Achillea New Vintage Red 32/32 Big Burly® Asclepias tuberosa 32/32 Big Burly® Achillea New Vintage Violet 32/32 Big Burly® Asclepias tuberosa 72/72 Big Burly® Achillea Paprika 32/32 Big Burly® Aster Balloon Mix 216/210 Pot-Ables™ Achillea Summer Drift 32/32 Big Burly® Aster Crown Mix 216/210 Pot-Ables™ Achillea Summer Pastels Mix 32/32 Big Burly® Aster Purple Dome 32/32 Big Burly® Achillea -

Volume 142 November, 2018 Firejail: Easy Sandbox on Pclinuxos
Volume 142 November, 2018 Firejail: Easy Sandbox On PCLinuxOS GIMP Tutorial: How To Apply A Sepia Tone Short Topix: Linux Is Changing The Face Of End-User Computing PCLinuxOS Family Member Spotlight: Martin Goose ANGRYsearch Microsoft Open Sources 60,000 Patents To Help Linux The Death Bell Tolls For G+ ms_meme's Nook: I Just Care For PCLinuxOS PCLinuxOS Recipe Corner: Mini Mozzarella Stuffed Turkey Zucchini Meatball Orechiette And more inside ... In This Issue... 3 From The Chief Editor's Desk 4 Firejail, Easy Sandbox On PCLinuxOS The PCLinuxOS name, logo and colors are the trademark of 7 Screenshot Showcase Texstar. 8 Short Topix: The PCLinuxOS Magazine is a monthly online publication containing PCLinuxOS-related materials. It is published Linux Is Changing The Face Of End-User Computing primarily for members of the PCLinuxOS community. The magazine staff is comprised of volunteers from the 15 GIMP Tutorial: How To Apply A Sepia Tone PCLinuxOS community. 17 Screenshot Showcase Visit us online at http://www.pclosmag.com 18 ms_meme's Nook: Booting From Both Sides This release was made possible by the following volunteers: 19 PCLinuxOS Family Member Spotlight: Martin Goose Chief Editor: Paul Arnote (parnote) Assistant Editor: Meemaw 21 Screenshot Showcase Artwork: Sproggy, Timeth, ms_meme, Meemaw Magazine Layout: Paul Arnote, Meemaw, ms_meme 22 Microsoft Open Sources Over 60,000 Patents HTML Layout: YouCanToo To Help Linux Staff: ms_meme CgBoy 23 Screenshot Showcase Meemaw YouCanToo Gary L. Ratliff, Sr. Pete Kelly 25 PCLinuxOS Recipe Corner Daniel Meiß-Wilhelm phorneker daiashi Khadis Thok 26 ANGRYsearch Alessandro Ebersol Smileeb 27 Screenshot Showcase Contributors: 28 The Death Bell Tolls For Google+ 30 ms_meme's Nook: I Just Care For PCLinuxOS 31 Screenshot Showcase The PCLinuxOS Magazine is released under the Creative 32 PCLinuxOS Bonus Recipe Corner Commons Attribution-NonCommercial-Share-Alike 3.0 Unported license. -

The Table of Equivalents / Replacements / Analogs of Windows Software in Linux
The table of equivalents / replacements / analogs of Windows software in Linux. Last update: 16.07.2003, 31.01.2005, 27.05.2005, 04.12.2006 You can always find the last version of this table on the official site: http://www.linuxrsp.ru/win-lin-soft/. This page on other languages: Russian, Italian, Spanish, French, German, Hungarian, Chinese. One of the biggest difficulties in migrating from Windows to Linux is the lack of knowledge about comparable software. Newbies usually search for Linux analogs of Windows software, and advanced Linux-users cannot answer their questions since they often don't know too much about Windows :). This list of Linux equivalents / replacements / analogs of Windows software is based on our own experience and on the information obtained from the visitors of this page (thanks!). This table is not static since new application names can be added to both left and the right sides. Also, the right column for a particular class of applications may not be filled immediately. In future, we plan to migrate this table to the PHP/MySQL engine, so visitors could add the program themselves, vote for analogs, add comments, etc. If you want to add a program to the table, send mail to winlintable[a]linuxrsp.ru with the name of the program, the OS, the description (the purpose of the program, etc), and a link to the official site of the program (if you know it). All comments, remarks, corrections, offers and bugreports are welcome - send them to winlintable[a]linuxrsp.ru. Notes: 1) By default all Linux programs in this table are free as in freedom.