First Confirmed Report of a Primary Freshwater Crab (Brachyura: Pseudothelphusidae) Associated with Bromeliads in the Neotropics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Classification of Living and Fossil Genera of Decapod Crustaceans
RAFFLES BULLETIN OF ZOOLOGY 2009 Supplement No. 21: 1–109 Date of Publication: 15 Sep.2009 © National University of Singapore A CLASSIFICATION OF LIVING AND FOSSIL GENERA OF DECAPOD CRUSTACEANS Sammy De Grave1, N. Dean Pentcheff 2, Shane T. Ahyong3, Tin-Yam Chan4, Keith A. Crandall5, Peter C. Dworschak6, Darryl L. Felder7, Rodney M. Feldmann8, Charles H. J. M. Fransen9, Laura Y. D. Goulding1, Rafael Lemaitre10, Martyn E. Y. Low11, Joel W. Martin2, Peter K. L. Ng11, Carrie E. Schweitzer12, S. H. Tan11, Dale Tshudy13, Regina Wetzer2 1Oxford University Museum of Natural History, Parks Road, Oxford, OX1 3PW, United Kingdom [email protected] [email protected] 2Natural History Museum of Los Angeles County, 900 Exposition Blvd., Los Angeles, CA 90007 United States of America [email protected] [email protected] [email protected] 3Marine Biodiversity and Biosecurity, NIWA, Private Bag 14901, Kilbirnie Wellington, New Zealand [email protected] 4Institute of Marine Biology, National Taiwan Ocean University, Keelung 20224, Taiwan, Republic of China [email protected] 5Department of Biology and Monte L. Bean Life Science Museum, Brigham Young University, Provo, UT 84602 United States of America [email protected] 6Dritte Zoologische Abteilung, Naturhistorisches Museum, Wien, Austria [email protected] 7Department of Biology, University of Louisiana, Lafayette, LA 70504 United States of America [email protected] 8Department of Geology, Kent State University, Kent, OH 44242 United States of America [email protected] 9Nationaal Natuurhistorisch Museum, P. O. Box 9517, 2300 RA Leiden, The Netherlands [email protected] 10Invertebrate Zoology, Smithsonian Institution, National Museum of Natural History, 10th and Constitution Avenue, Washington, DC 20560 United States of America [email protected] 11Department of Biological Sciences, National University of Singapore, Science Drive 4, Singapore 117543 [email protected] [email protected] [email protected] 12Department of Geology, Kent State University Stark Campus, 6000 Frank Ave. -

Epilobocera Wetherbeei, a New Species of Freshwater Crab (Decapoda: Brachyura: Pseudothelphusidae) from Hispaniola Gilberto Rodr
PROCEEDINGS OF THE BIOLOGICAL SOCIETY OF WASHINGTON 108(l):76-83. 1995. Epilobocera wetherbeei, a new species of freshwater crab (Decapoda: Brachyura: Pseudothelphusidae) from Hispaniola Gilberto Rodriguez and Austin B. Williams (GR) Instituto Venezolano de Investigaciones Cientificas, Apartado 21827, Caracas 1020 A, Venezuela; (ABW) National Marine Fisheries Service Systematics Laboratory, National Museum of Natural History, Smithsonian Institution, Washington, D.C. 20560, U.S.A. Abstract.—Epilobocera wetherbeei, a new species of pseudothelphusid crab, is described from the Dominican Republic. The species can be easily distin guished from other species of Epilobocera by its small size, absence of antero lateral spinulation and characters of the first male gonopods. SEM micropho- tographs of the first and second male gonopods of E. wetherbeei, E. sinuatifrons, E. haytensis and E. gertraudae are provided. The apex of the second male gonopod in all species of Epilobocera studied is hollow, transversely truncate, with a prominent internal rib provided with spines. These characters distinctly separate Epilobocera from species in the subfamily Pseudothelphusinae where the gonopodal apex is spoon-shaped and provided with long setae. The new observations are incorporated into amended diagnoses of the subfamilies Epi- lobocerinae and Pseudothelphusinae. The genus Epilobocera Stimpson, 1860, less it can be shown to be a synonym of E. comprises six species of freshwater crabs re armata."' The genus has been the object of stricted to the Greater Antilles and some recent revisions by Chace & Hobbs (1969), nearby islands: E. sinuatifrons A. Milne Ed Pretzmann (1972) and Rodriguez (1982). wards, 1866, inhabits Puerto Rico and Saint Due to the accessibility and small area of Croix Island; E. -

49 a New Genus and Species of Tree-Climbing Crab
THE RAFFLES BULLETIN OF ZOOLOGY 2003 THE RAFFLES BULLETIN OF ZOOLOGY 2003 51(1): 49-59 © National University of Singapore A NEW GENUS AND SPECIES OF TREE-CLIMBING CRAB (CRUSTACEA: BRACHYURA: SESARMIDAE) FROM TAIWAN WITH NOTES ON ITS ECOLOGY AND LARVAL MORPHOLOGY Christoph D. Schubart Biologie 1 (Zoologie), Universität Regensburg, 93040 Regensburg, Germany Email: [email protected] Hung-Chang Liu Institute of Zoology, Academia Sinica, Nankang, Taipei 115 Taiwan, R.O.C. José A. Cuesta Instituto de Ciencias Marinas de Andalucía (CSIC), Campus Río San Pedro, Apdo. Oficial, 11510 Puerto Real, Cádiz, Spain ABSTRACT. – A new genus and species of sesarmid crab, Scandarma lintou, is described from Taiwan. This crab has a semi-terrestrial habit: adults and juveniles thrive in wind-protected and vegetated habitats in close vicinity of fresh water and up to one kilometer from the sea. In southern Taiwan, this species was most commonly found hiding in leaf axils or climbing on the thorny leaves of the screw pine Pandanus odoratissimus Linnaeus. During the reproductive season (June to January), ovigerous females migrate to estuaries, where small and free-swimming pelagic larvae are released into the brackish waters and probably washed into the sea. Morphologically, this species is superficially similar to species of other semi-terrestrial sesarmid genera, but differs from these taxa by the shape of the anterolateral carapace region, by the markedly flattened fingers, by the presence of a row of tubercles on the dorsal border of the dactylus, a row of ventral spines on the pollex, and a granular ridge on the dorsal face of the palm. -

Molecular Phylogeny, Taxonomy, and Evolution of Nonmarine Lineages Within the American Grapsoid Crabs (Crustacea: Brachyura) Christoph D
Molecular Phylogenetics and Evolution Vol. 15, No. 2, May, pp. 179–190, 2000 doi:10.1006/mpev.1999.0754, available online at http://www.idealibrary.com on Molecular Phylogeny, Taxonomy, and Evolution of Nonmarine Lineages within the American Grapsoid Crabs (Crustacea: Brachyura) Christoph D. Schubart*,§, Jose´ A. Cuesta†, Rudolf Diesel‡, and Darryl L. Felder§ *Fakulta¨tfu¨ r Biologie I: VHF, Universita¨ t Bielefeld, Postfach 100131, 33501 Bielefeld, Germany; †Departamento de Ecologı´a,Facultad de Biologı´a,Universidad de Sevilla, Apdo. 1095, 41080 Sevilla, Spain; ‡Max-Planck-Institut fu¨ r Verhaltensphysiologie, Postfach 1564, 82305 Starnberg, Germany; and §Department of Biology and Laboratory for Crustacean Research, University of Louisiana at Lafayette, Lafayette, Louisiana 70504-2451 Received January 4, 1999; revised November 9, 1999 have attained lifelong independence from the sea (Hart- Grapsoid crabs are best known from the marine noll, 1964; Diesel, 1989; Ng and Tan, 1995; Table 1). intertidal and supratidal. However, some species also The Grapsidae and Gecarcinidae have an almost inhabit shallow subtidal and freshwater habitats. In worldwide distribution, being most predominant and the tropics and subtropics, their distribution even species rich in subtropical and tropical regions. Over- includes mountain streams and tree tops. At present, all, there are 57 grapsid genera with approximately 400 the Grapsoidea consists of the families Grapsidae, recognized species (Schubart and Cuesta, unpubl. data) Gecarcinidae, and Mictyridae, the first being subdi- and 6 gecarcinid genera with 18 species (Tu¨ rkay, 1983; vided into four subfamilies (Grapsinae, Plagusiinae, Tavares, 1991). The Mictyridae consists of a single Sesarminae, and Varuninae). To help resolve phyloge- genus and currently 4 recognized species restricted to netic relationships among these highly adaptive crabs, portions of the mitochondrial genome corresponding the Indo-West Pacific (P. -

Freshwater Crabs As Predators and Prey: the Case of Ptychophallus Uncinatus Campos & Lemaitre, 1999 (Brachyura, Pseudothelphusidae) from Costa Rica, Central America
Latin American Journal of Aquatic Research, 47(1): 18Ptychophallus-26, 2019 uncinatus from Costa Rica 1 DOI: 10.3856/vol47-issue1-fulltext-3 Research Article Freshwater crabs as predators and prey: the case of Ptychophallus uncinatus Campos & Lemaitre, 1999 (Brachyura, Pseudothelphusidae) from Costa Rica, Central America Ingo S. Wehrtmann1,2, Dayan Hernández-Díaz3 & Neil Cumberlidge4 1Museo de Zoología, Escuela de Biología, Universidad de Costa Rica, San José, Costa Rica 2Unidad de Investigación Pesquera y Acuicultura (UNIP), Centro de Investigación en Ciencias del Mar y Limnología (CIMAR), Universidad de Costa Rica, San José, Costa Rica 3Escuela de Biología, Universidad Latina de Costa Rica, Sede San Pedro, Costa Rica 4Department of Biology, Northern Michigan University, Marquette, MI, USA Corresponding author: Ingo S. Wehrtmann ([email protected]) ABSTRACT. Primary freshwater crabs are an important component of the food web in aquatic ecosystems, but our knowledge about the role of these decapods as predators and as prey is far from complete. Here we report observations of the feeding habits of the pseudothelphusid crab Ptychophallus uncinatus Campos & Lemaitre, 1999, made in 2013 during exploratory observations after sunset in the dusk and darkness of the early evening within the Veragua Rainforest Research & Adventure Park, Limón Province, in the Atlantic drainage of Costa Rica. We observed a case of cannibalism where an adult P. uncinatus was feeding on a smaller crab. Furthermore, P. uncinatus was observed to prey on an insect larva, a frog, and a lizard on three separate occasions. Additionally, a spider of the family Ctenidae was discovered feeding on a specimen of P. -

Recent Advances in the Biology of the Neotropical Freshwater Crab Family Pseudothelphusidae (Crustacea, Decapoda, Brachyura)
Recent advances in the biology of the Neotropical freshwater crab family Pseudothelphusidae (Crustacea, Decapoda, Brachyura) Gilberto Rodríguez 1 & Célio Magalhães 2 1 Centro de Ecología, Instituto Venezolano de Investigaciones Científicas, Caracas, Venezuela. (In memoriam) 2 Author for correspondence. Instituto Nacional de Pesquisas do Amazônia, Caixa postal 478, 69011-970 Manaus, Amazonas, Brasil. Research fellow of the CNPq. E-mail: [email protected] ABSTRACT. Pseudothelphusidae is a well diversified group of Neotropical freshwater crabs currently compris- ing 40 genera and at least 255 species and subspecies. The biology of these crabs has been an active field of research in the last 20 years. The aim of the present contribution is to discuss the significance of the new knowledge on the biology of these freshwater crabs after September 1992, to stress the interconnection of the diverse lines of research and at the same time to suggest promising new lines of investigation. All taxa described from September 1992 to October 2004 are listed, including one genus, one subgenus, 62 species and five subspecies. The implications of this new knowledge on the taxonomy, systematic and biogeography of the family are commented. KEY WORDS. Biodiversity, biogeography, Neotropical region, taxonomy. RESUMO. Avanços recentes no estudo da biologia dos caranguejos de água doce neotropicais da família Pseudothelphusidae (Crustaceaustacea, Decapodapoda, Brachyura). Pseudothelphusidae é um grupo bem diversificado de caranguejos de água doce neotropicais que compreende atualmente 40 gêneros e pelo menos 255 espécies e subespécies. A biologia desses caranguejos vem sendo um ativo campo de pesquisa nos últimos 20 anos. O objetivo desta contribuição é discutir o significado do conhecimento adquirido sobre a biologia desses caran- guejos dulcícolas após setembro de 1992, enfatizar a relação das diversas linhas de pesquisa e, ao mesmo tempo, sugerir novas linhas promissoras de investigação. -
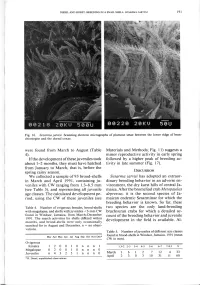
(Table 4). If the Development of These Juveniles Took About 1-2 Months
DIESEL AND HORST: BREEDING IN A SNAIL SHELL: SESARMA JARVISI 191 Fig. 16. Sesarma jarvisi. Scanning electron micrographs of plumose setae between the lower ridge of bran- chiostegite and the dorsal coxae. were found from March to August (Table Materials and Methods; Fig. 11) suggests a 4). minor reproductive activity in early spring If the development of these juveniles took followed by a higher peak of breeding ac- about 1-2 months, they must have hatched tivity in late summer (Fig. 17). from January to March, that is, before the spring rainy season. DISCUSSION We collected a sample of 93 brood-shells Sesarma jarvisi has adopted an extraor- in March and April 1991, containing ju- dinary breeding behavior in an adverse en- veniles with CW ranging from 1.3-8.5 mm vironment, the dry karst hills of central Ja- (see Table 5), and representing all juvenile maica. After the bromeliad crab Metopaulias age classes. The calculated development pe- depressus, it is the second species of Ja- riod, using the CW of these juveniles (see maican endemic Sesarminae for which the breeding behavior is known. So far, these Table 4. Number of ovigerous females, brood-shells two species are the only land-breeding with megalopae, and shells with juveniles <3-mm CW brachyuran crabs for which a detailed ac- found in Windsor, Jamaica, from March-December count of the breeding behavior and juvenile 1991. The search activities for shells differed within months, and brood-shells were only occasionally development in the field is available. Al- searched for in August and December, n = no obser- vations. -

Potential and Pitfalls of Low Coverage Genome Surveys for Evolutionary Biology
Exploring Pandora’s Box: Potential and Pitfalls of Low Coverage Genome Surveys for Evolutionary Biology Florian Leese1,2*, Philipp Brand1., Andrey Rozenberg1., Christoph Mayer4, Shobhit Agrawal3, Johannes Dambach4, Lars Dietz1, Jana S. Doemel1, William P. Goodall-Copstake2, Christoph Held3, Jennifer A. Jackson2, Kathrin P. Lampert1, Katrin Linse2, Jan N. Macher1, Jennifer Nolzen1, Michael J. Raupach5, Nicole T. Rivera6, Christoph D. Schubart6, Sebastian Striewski1, Ralph Tollrian1, Chester J. Sands2 1 Ruhr University Bochum, Department of Animal Ecology, Evolution and Biodiversity, Bochum, Germany, 2 British Antarctic Survey, High Cross, Madingley Road, Cambridge, United Kingdom, 3 Alfred Wegener Institute for Polar and Marine Research, Functional Ecology, Bremerhaven, Germany, 4 Zoologisches Forschungsmuseum Alexander Koenig, Bonn, Germany, 5 Senckenberg am Meer, German Center for Marine Biodiversity Research, Molecular Taxonomy Group, Wilhelmshaven, Germany, 6 University of Regensburg, Biologie 1, Department of Evolution, Behavior and Genetics, Regensburg, Germany Abstract High throughput sequencing technologies are revolutionizing genetic research. With this ‘‘rise of the machines’’, genomic sequences can be obtained even for unknown genomes within a short time and for reasonable costs. This has enabled evolutionary biologists studying genetically unexplored species to identify molecular markers or genomic regions of interest (e.g. micro- and minisatellites, mitochondrial and nuclear genes) by sequencing only a fraction of the genome. However, when using such datasets from non-model species, it is possible that DNA from non-target contaminant species such as bacteria, viruses, fungi, or other eukaryotic organisms may complicate the interpretation of the results. In this study we analysed 14 genomic pyrosequencing libraries of aquatic non-model taxa from four major evolutionary lineages. -

Duffy 2010 Encyclopedia of Animal Behavior-1.Pdf
This article was originally published in the Encyclopedia of Animal Behavior published by Elsevier, and the attached copy is provided by Elsevier for the author's benefit and for the benefit of the author's institution, for non- commercial research and educational use including without limitation use in instruction at your institution, sending it to specific colleagues who you know, and providing a copy to your institution’s administrator. All other uses, reproduction and distribution, including without limitation commercial reprints, selling or licensing copies or access, or posting on open internet sites, your personal or institution’s website or repository, are prohibited. For exceptions, permission may be sought for such use through Elsevier's permissions site at: http://www.elsevier.com/locate/permissionusematerial Duffy J.E. (2010) Crustacean Social Evolution. In: Breed M.D. and Moore J., (eds.) Encyclopedia of Animal Behavior, volume 1, pp. 421-429 Oxford: Academic Press. © 2010 Elsevier Ltd. All rights reserved. Author's personal copy Crustacean Social Evolution J. E. Duffy, Virginia Institute of Marine Science, Gloucester Point, VA, USA ã 2010 Elsevier Ltd. All rights reserved. Introduction shed periodically during growth. Each of the segments in the primitive ancestral crustacean body bore a pair of The Crustacea represent one of the most spectacular appendages, which have been modified during the evolu- evolutionary radiations in the animal kingdom, whether tion of the various crustacean groups into a wide range of measured by species richness or diversity in morphology structures used in feeding, locomotion, sensation, and or lifestyles. Its members range from microscopic mites of communication. -

Generating Genomic Resources for Two Crustacean Species and Their Application to the Study of White Spot Disease
Generating genomic resources for two crustacean species and their application to the study of White Spot Disease Submitted by Bas Verbruggen To the University of Exeter as a thesis for the degree of Doctor of Philosophy in Biological Sciences, September 2016 This thesis is available for Library use on the understanding that it is copyright material and that no quotation from the thesis may be published without proper acknowledgement I certify that all material in this thesis which is not my own work has been identified and that no material has previously been submitted and approved for the award of a degree by this or any other University. Signature: ……………………………………(Bas Verbruggen) Abstract Over the last decades the crustacean aquaculture sector has been steadily growing, in order to meet global demands for its products. A major hurdle for further growth of the industry is the prevalence of viral disease epidemics that are facilitated by the intense culture conditions. A devastating virus impacting on the sector is the White Spot Syndrome Virus (WSSV), responsible for over US $ 10 billion in losses in shrimp production and trade. The Pathogenicity of WSSV is high, reaching 100 % mortality within 3-10 days in penaeid shrimps. In contrast, the European shore crab Carcinus maenas has been shown to be relatively resistant to WSSV. Uncovering the basis of this resistance could help inform on the development of strategies to mitigate the WSSV threat. C. maenas has been used widely in studies on ecotoxicology and host-pathogen interactions. However, like most aquatic crustaceans, the genomic resources available for this species are limited, impairing experimentation. -

Download Preprint
Biological Reviews Macroecology of parent al care in arthropods: higher mortality risk leads to higher benefits of offspring protection in tropical climates Journal:For Biological Review Reviews Only Manuscript ID BRV-04-2016-0076.R2 Manuscript Type: Original Article Date Submitted by the Author: n/a Complete List of Authors: Santos, Eduardo; Universidade de Sao Paulo, Departamento de Zoologia Bueno, Pedro; Universidade de Sao Paulo, Departamento de Ecologia Gilbert, James; University of Hull, School of Biological, Biomedical & Environmental Sciences Machado, Glauco; Universidade de Sao Paulo, Departamento de Ecologia abiotic factors, biotic interactions, evapotranspiration, egg attendance, egg Keywords: coating, meta-regression, nest, parasitism, parental removal, predation Page 1 of 60 Biological Reviews 1 2 3 1 Macroecology of parental care in arthropods: higher mortality 4 5 6 2 risk leads to higher benefits of offspring protection in tropical 7 8 9 3 climates 10 11 12 4 13 14 5 Eduardo S. A. Santos 1, 2, *, Pedro P. Bueno 1, James D. J. Gilbert 3 and Glauco 15 16 1 17 6 Machado 18 For Review Only 19 7 20 21 8 1LAGE do Departamento de Ecologia, Instituto de Biociências, Universidade de São Paulo, 22 23 24 9 Rua do Matão, trav. 14, no 321, Cidade Universitária, 05508-090, São Paulo, SP, Brazil 25 2 26 10 BECO do Departamento de Zoologia, Instituto de Biociências, Universidade de São Paulo, 27 28 11 Rua do Matão, trav. 14, no 321, Cidade Universitária, 05508-090, São Paulo, SP, Brazil 29 30 12 3School of Environmental Sciences, University of Hull, Cottingham Rd, Hull HU6 7RX, UK 31 32 33 13 34 35 14 36 37 15 Running title : Macroecology of parental care in arthropods 38 39 16 40 41 17 42 43 * 44 18 Author for correspondence (E-mail: [email protected]; Tel.: +55 (11) 3091-0989). -

The Reclassification of Brachyuran Crabs (Crustacea: Decapoda: Brachyura)
NAT. CROAT. VOL. 14 Suppl. 1 1¿159 ZAGREB June 2005 THE RECLASSIFICATION OF BRACHYURAN CRABS (CRUSTACEA: DECAPODA: BRACHYURA) ZDRAVKO [TEV^I] Laco Sercio 19, HR-52210 Rovinj, Croatia [tev~i}, Z.: The reclassification of brachyuran crabs (Crustacea: Decapoda: Brachyura). Nat. Croat., Vol. 14, Suppl. 1, 1–159, 2005, Zagreb. A reclassification of brachyuran crabs (Crustacea: Decapoda: Brachyura) including a re-ap- praisal of their whole systematics, re-assessment of the systematic status and position of all extant and extinct suprageneric taxa and their redescription, as well as a description of new taxa, has been undertaken. A great number of new higher taxa have been established and the majority of higher taxa have had their systematic status and position changed. Key words: brachyuran crabs, Crustacea, Decapoda, Brachyura, systematics, revision, reclassifi- cation. [tev~i}, Z.: Reklasifikacija kratkorepih rakova (Crustacea: Decapoda: Brachyura). Nat. Croat., Vol. 14, Suppl. 1, 1–159, 2005, Zagreb. Reklasifikacija kratkorepih rakova (Crustacea: Decapoda: Brachyura) odnosi se na preispitivanje cjelokupnog njihovog sustava, uklju~uju}i preispitivanje sistematskog statusa i polo`aja sviju recentnih i izumrlih svojti iznad razine roda kao i njihove ponovne opise. Uspostavljeno je mnogo novih vi{ih svojti, a ve}ini je izmijenjen sistematski status i polo`aj. Klju~ne rije~i: kratkorepi raci, Crustacea, Decapoda, Brachyura, sistematika, revizija, reklasi- fikacija INTRODUCTION Brachyuran crabs (Crustacea: Decapoda: Brachyura) are one of the most diverse animal groups at the infra-order level. They exhibit an outstanding diversity in the numbers of extant and extinct taxa at all categorical levels. Recently, especially dur- ing the past several decades, judging from the number of publications and new taxa described, the knowledge of their systematics has increased rapidly.