Gharial Nesting in a Reservoir Is Limited by Reduced River Flow and by Increased Bank Vegetation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Geomorphological Field Guide Book CHAMBAL BADLANDS
Geomorphological Field Guide Book on CHAMBAL BADLANDS By Editor H.S. Sharma* & Amal Kar Padmini Pani** Kolkata *Formerly of Rajasthan University, Jaipur ** Jawaharlal Nehru University, Formerly at Central Arid Zone Research New Delhi Institute (CAZRI), Jodhpur Published on the occasion of New Delhi, 2017 Published by: Indian Institute of Geomorphologists (IGI), Allahabad On the occasion of: 9th International Conference on Geomorphology of the International Association of Geomorphologists (IAG), New Delhi (6-11 November, 2017) Citation: Sharma, H.S. and Pani, P. 2017. Geomorphological Field Guide Book on Chambal Badlands (Edited by Amal Kar). Indian Institute of Geomorphologists, Allahabad. 1 Fig. 1. Image-map of India, showing some places of interest for the 9th International Conference on Geomorphology, 2017 (Map prepared by A. Kar through processing of relevant ETM+ FCC mosaics and SRTM 1km DEM, both sourced from the US Geological Survey site). Boundaries are approximate. 2 Geomorphological Field Guide Book on Chambal Badlands Itinerary Day Places from - to Stay Day 1 Arrival at Agra Agra Visit in and around Agra Day 2 Field visit to Sahso, Bindwa Khurd and Agra back to Agra Day 3 Field visit to Emiliya and back to Agra Depart from Agra 3 4 A. CHAMBAL BADLANDS: AN INTRODUCTION Land degradation is considered to be one of the most severe global environmental challenges (Eswaran et al., 2001; Lal, 2001; Scherr and Yadav, 2001). It has numerous economic, social and ecological consequences. Land degradation is also an important geomorphic process in many parts of the world and in a range of landscapes. Its causal determinants, in terms of local specificities, are yet to be understood fully (Lambin et al., 2003, 2009). -

Chowkidar 10 04.Pdf
Registered Charity 273422 ISSN 0141-6588 CttOWKlDAR Volume 1O Number 4 Autum 2004 Editor: Dr. Rosie Llewellyn-Jones British Association For Cemeteries In South Asia (BACSA) HARRY ANDERSON'S STORY President Chairman The Rt. Hon. Lord Rees , QC Mr. A . J . Farrington Earlier this year BACSA member Virginia van der Lande returned from a visit to India, where she has long family ties. Colonel John Cumming Council Executive Committee Anderson of the Madras Engineers was her mother's paternal grandfather Sir Nicholas Barrington , KCMG, CVO Dr. R. J. Bingle (Records archive) Sir William Benyon Mr. H. C. Q. Brownrigg and there is a relationship with the great General Sir James Outram too. Sir Charles Frossard, KBE Dr. W. F. Crawley (PRO & Book project) Another connection, Lieutenant Robert Anderson, published his Personal Mr. P.A. Leggatt, MBE Mr. D. H. Doble Journal of the Siege of Lucknow in 1858, a year after the terrible events of Mr. G.Shaw Miss S. M. Farrington the Mutiny. 'While in Calcutta' Dr van der Lande tells us 'I played truant The Rt. Hon. The Viscount Slim, OBE Mrs. M. Hywel -Jones (Guide Book project) Mr. H. M. Stokes Mr. D. W. Mahoney for a day to visit the Anglican cemetery at Krishnagar where BACSA's 1982 list Lady Wade-Gery Mr. M. J. Murphy told me of the tomb of an uncle of Colonel John Cumming Anderson. This was Mr. T. C. Wilkinson, MBE (Publications) Mrs. V. W. Robinson (acting Events Officer) Captain Henry (Harry) Anderson of the 12th Native Infantry, who died from Mr. -

LIST of INDIAN CITIES on RIVERS (India)
List of important cities on river (India) The following is a list of the cities in India through which major rivers flow. S.No. City River State 1 Gangakhed Godavari Maharashtra 2 Agra Yamuna Uttar Pradesh 3 Ahmedabad Sabarmati Gujarat 4 At the confluence of Ganga, Yamuna and Allahabad Uttar Pradesh Saraswati 5 Ayodhya Sarayu Uttar Pradesh 6 Badrinath Alaknanda Uttarakhand 7 Banki Mahanadi Odisha 8 Cuttack Mahanadi Odisha 9 Baranagar Ganges West Bengal 10 Brahmapur Rushikulya Odisha 11 Chhatrapur Rushikulya Odisha 12 Bhagalpur Ganges Bihar 13 Kolkata Hooghly West Bengal 14 Cuttack Mahanadi Odisha 15 New Delhi Yamuna Delhi 16 Dibrugarh Brahmaputra Assam 17 Deesa Banas Gujarat 18 Ferozpur Sutlej Punjab 19 Guwahati Brahmaputra Assam 20 Haridwar Ganges Uttarakhand 21 Hyderabad Musi Telangana 22 Jabalpur Narmada Madhya Pradesh 23 Kanpur Ganges Uttar Pradesh 24 Kota Chambal Rajasthan 25 Jammu Tawi Jammu & Kashmir 26 Jaunpur Gomti Uttar Pradesh 27 Patna Ganges Bihar 28 Rajahmundry Godavari Andhra Pradesh 29 Srinagar Jhelum Jammu & Kashmir 30 Surat Tapi Gujarat 31 Varanasi Ganges Uttar Pradesh 32 Vijayawada Krishna Andhra Pradesh 33 Vadodara Vishwamitri Gujarat 1 Source – Wikipedia S.No. City River State 34 Mathura Yamuna Uttar Pradesh 35 Modasa Mazum Gujarat 36 Mirzapur Ganga Uttar Pradesh 37 Morbi Machchu Gujarat 38 Auraiya Yamuna Uttar Pradesh 39 Etawah Yamuna Uttar Pradesh 40 Bangalore Vrishabhavathi Karnataka 41 Farrukhabad Ganges Uttar Pradesh 42 Rangpo Teesta Sikkim 43 Rajkot Aji Gujarat 44 Gaya Falgu (Neeranjana) Bihar 45 Fatehgarh Ganges -
Current Condition of the Yamuna River - an Overview of Flow, Pollution Load and Human Use
Current condition of the Yamuna River - an overview of flow, pollution load and human use Deepshikha Sharma and Arun Kansal, TERI University Introduction Yamuna is the sub-basin of the Ganga river system. Out of the total catchment’s area of 861404 sq km of the Ganga basin, the Yamuna River and its catchment together contribute to a total of 345848 sq. km area which 40.14% of total Ganga River Basin (CPCB, 1980-81; CPCB, 1982-83). It is a large basin covering seven Indian states. The river water is used for both abstractive and in stream uses like irrigation, domestic water supply, industrial etc. It has been subjected to over exploitation, both in quantity and quality. Given that a large population is dependent on the river, it is of significance to preserve its water quality. The river is polluted by both point and non-point sources, where National Capital Territory (NCT) – Delhi is the major contributor, followed by Agra and Mathura. Approximately, 85% of the total pollution is from domestic source. The condition deteriorates further due to significant water abstraction which reduces the dilution capacity of the river. The stretch between Wazirabad barrage and Chambal river confluence is critically polluted and 22km of Delhi stretch is the maximum polluted amongst all. In order to restore the quality of river, the Government of India (GoI) initiated the Yamuna Action Plan (YAP) in the1993and later YAPII in the year 2004 (CPCB, 2006-07). Yamuna river basin River Yamuna (Figure 1) is the largest tributary of the River Ganga. The main stream of the river Yamuna originates from the Yamunotri glacier near Bandar Punch (38o 59' N 78o 27' E) in the Mussourie range of the lower Himalayas at an elevation of about 6320 meter above mean sea level in the district Uttarkashi (Uttranchal). -

Master of the Marsh Information for Cart
Mighty MikeMighty Mike:Mike: The Master of the Marsh A story of when humans and predators meet Alligators are magnificent predators that have lived for millions of years and demonstrate amazing adaptations for survival. Their “recent” interaction with us demonstrates the importance of these animals and that we have a vital role to play in their survival. Primary Exhibit Themes: 1. American Alligators are an apex predator and a keystone species of wetland ecosystems throughout the southern US, such as the Everglades. 2. Alligators are an example of a conservation success story. 3. The wetlands that alligators call home are important ecosystems that are in need of protection. Primary Themes and Supporting Facts 1. Alligators are an apex predator and, thus, a keystone species of wetland ecosystems throughout the southern US, such as the Everglades. a. The American Alligator is known as the “Master of the Marsh” or “King of the Everglades” b. What makes a great predator? Muscles, Teeth, Strength & Speed i. Muscles 1. An alligator has the strongest known bite of any land animal – up to 2,100 pounds of pressure. 2. Most of the muscle in an alligators jaw is intended for biting and gripping prey. The muscles for opening their jaws are relatively weak. This is why an adult man can hold an alligators jaw shut with his bare hands. Don’t try this at home! ii. Teeth 1. Alligators have up to 80 teeth. 2. Their conical teeth are used for catching the prey, not tearing it apart. 3. They replace their teeth as they get worn and fall out. -

Violations in the Name of Conservation “What Crime Had I Committed by Putting My Feet on the Land That I Own?”
VIOLATIONS IN THE NAME OF CONSERVATION “WHAT CRIME HAD I COMMITTED BY PUTTING MY FEET ON THE LAND THAT I OWN?” Amnesty International is a movement of 10 million people which mobilizes the humanity in everyone and campaigns for change so we can all enjoy our human rights. Our vision is of a world where those in power keep their promises, respect international law and are held to account. We are independent of any government, political ideology, economic interest or religion and are funded mainly by our membership and individual donations. We believe that acting in solidarity and compassion with people everywhere can change our societies for the better. © Amnesty International 2021 Except where otherwise noted, content in this document is licensed under a Creative Commons Cover photo: Illustration by Colin Foo (attribution, non-commercial, no derivatives, international 4.0) licence. Photo: Chitwan National Park, Nepal. © Jacek Kadaj via Getty Images https://creativecommons.org/licenses/by-nc-nd/4.0/legalcode For more information please visit the permissions page on our website: www.amnesty.org Where material is attributed to a copyright owner other than Amnesty International this material is not subject to the Creative Commons licence. First published in 2021 by Amnesty International Ltd Peter Benenson House, 1 Easton Street London WC1X 0DW, UK Index: ASA 31/4536/2021 Original language: English amnesty.org CONTENTS 1. EXECUTIVE SUMMARY 5 1.1 PROTECTING ANIMALS, EVICTING PEOPLE 5 1.2 ANCESTRAL HOMELANDS HAVE BECOME NATIONAL PARKS 6 1.3 HUMAN RIGHTS VIOLATIONS BY THE NEPAL ARMY 6 1.4 EVICTION IS NOT THE ANSWER 6 1.5 CONSULTATIVE, DURABLE SOLUTIONS ARE A MUST 7 1.6 LIMITED POLITICAL WILL 8 2. -

Assessment of Minimum Water Flow Requirements of Chambal River
Assessment of minimum water flow requirements of Chambal River in the context of Gharial (Gavialis gangeticus) and Gangetic Dolphin (Platanista gangetica) conservation Study Report April 2011 Assessmentofminimumwaterflowrequirements ofChambalRiverinthecontextofGharial(Gavialis gangeticus)andGangeticDolphin(Platanista gangetica)conservation StudyReport April2011 Contributors:SyedAinulHussain,R.K.Shrama,NiladriDasguptaandAngshumanRaha. CONTENTS Executivesummary 1 1. Background 3 2. Introduction 3 3. TheChambalriver 3 4. Existingandproposedwaterrelatedprojects 5 5. TheNationalChambalSanctuary 8 6. Thegharial(Gavialisgangeticus) 8 7. TheGangeticdolphin(Platanistagangetica) 9 8. Objectivesofassessment 10 9. Methodsofassessment 12 10. Results 13 11. Discussion 20 12. References 22 13. AppendixI–IV 26 AssessmentofminimumwaterflowrequirementsofChambalRiver ʹͲͳͳ EXECUTIVESUMMARY The Chambal River originates from the summit of Janapav hill of the Vindhyan range at an altitudeof854mabovethemslat22027’Nand75037’EinMhow,districtIndore,Madhya Pradesh.Theriverhasacourseof965kmuptoitsconfluencewiththeYamunaRiverinthe EtawahdistrictofUttarPradesh.ItisoneofthelastremnantriversinthegreaterGangesRiver system, which has retained significant conservation values. It harbours the largest gharial population of the world and high density of the Gangetic dolphin per river km. Apart from these,themajorfaunaoftheRiverincludesthemuggercrocodile,smoothͲcoatedotter,seven speciesoffreshwaterturtles,and78speciesofwetlandbirds.Themajorterrestrialfaunaofthe -
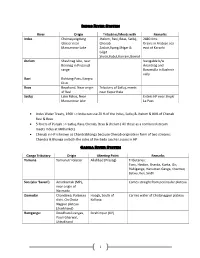
1 Indus River System River Origin Tributries/Meets with Remarks
Indus River System River Origin Tributries/Meets with Remarks Indus Chemayungdung Jhelum, Ravi, Beas, Satluj, 2880 Kms Glacier near Chenab Drains in Arabian sea Mansarovar Lake Zaskar,Syang,Shigar & east of Karachi Gilgit Shyok,Kabul,Kurram,Gomal Jhelum Sheshnag lake, near Navigable b/w Beninag in Pirpanjal Anantnag and range Baramulla in Kashmir vally Ravi Rohtang Pass, Kangra Distt. Beas Beaskund, Near origin Tributary of Satluj, meets of Ravi near Kapurthala Satluj Lake Rakas, Near Enters HP near Shipki Mansarovar lake La Pass Indus Water Treaty, 1960 :-> India can use 20 % of the Indus, Satluj & Jhelum & 80% of Chenab Ravi & Beas 5 Rivers of Punjab :-> Satluj, Ravi, Chenab, Beas & Jhelum ( All these as a combined stream meets Indus at Mithankot) Chenab in HP is known as Chandrabhanga because Chenab originate in form of two streams: Chandra & Bhanga on both the sides of the Bada Laccha La pass in HP. Ganga River System Ganga Tributary Origin Meeting Point Remarks Yamuna Yamunotri Glaciar Allahbad (Prayag) Tributaries: Tons, Hindon, Sharda, Kunta, Gir, Rishiganga, Hanuman Ganga, Chambal, Betwa, Ken, Sindh Son (aka ‘Savan’) Amarkantak (MP), Comes straight from peninsular plateau near origin of Narmada Damodar Chandawa, Palamau Hoogli, South of Carries water of Chotanagpur plateau distt. On Chota Kolkata Nagpur plateau (Jharkhand) Ramganga: Doodhatoli ranges, Ibrahimpur (UP) Pauri Gharwal, Uttrakhand 1 Gandak Nhubine Himal Glacier, Sonepur, Bihar It originates as ‘Kali Gandak’ Tibet-Mustang border Called ‘Narayani’ in Nepal nepal Bhuri Gandak Bisambharpur, West Khagaria, Bihar Champaran district Bhagmati Where three headwater streams converge at Bāghdwār above the southern edge of the Shivapuri Hills about 15 km northeast of Kathmandu Kosi near Kursela in the Formed by three main streams: the Katihar district Tamur Koshi originating from Mt. -

LIFELINES of INDIA's CIVILISATION in India, a River Is a Mini-Cosmos In
LIFELINES OF INDIA’S CIVILISATION In India, a river is a mini-cosmos in concept. Every river is a mother deity who spawns mythology, art, dance, music, architecture, history and spirituality. Each one has a clear identity, appearance, value, style and spirit just like a beautiful woman. In every age, diverse human communities have reinvented themselves on river-banks with fascinating nuances…. ‘Her shimmering gold-and-white garments dazzle like a thousand suns. The jewels in her crown shine like the crescent moon. Her smiling face lights up the whole world. In her hands, she carries a pot of nectar, a symbol of immortality. Her lotus-fresh presence brings a sense of purity and joy to all beings….’. At first glance, this reads like an over-the-top flowery description of a beautiful woman coined by some besotted lover. But to those conversant with the fascinating river-lore of India, this is the mythical portrayal of the River Ganga, written by Sage Valmiki, author of India’s immortal epic Ramayan. It describes the celestial Ganga as she descends from the heavens to the earth to bring salvation to mankind. This story, known asGangavataran, is such a fundamental tenet of Indian culture that it has held countless generations of Indians in awe for millenniums. The Ganga, arguably the most picturised and written-about river in the world, has been called the Mother of India’s Spirituality and has been immortalized in sculpture, art, literature, poetry, music and dance. Following her descent to the mortal world to sanctify human efforts to attain salvation, the Ganga is perceived as mokshdayini, the Mother Goddess whose waters bring relief from sin, sorrow and suffering. -

Groundwater Research in NEPAL for Tiger Conservation
GROUNDWATER RESEARCH IN NEPAL FOR TIGER CONSERVATION A reconnaissance study to groundwater dynamics in an alluvial mega-fan in Bardiya National Park (Terai), focusing on the interaction between groundwater and the Karnali river. Author: Hanne. Berghuis. MSc. Thesis. Program: Earth, Surface and Water at Utrecht University. 1st Supervisor: Prof. Dr. Jasper Griffioen. 2nd Supervisor: Dr. Derek Karssenberg. Date: 28-06-2019. Student No.: 6190987. Contact: [email protected]. Photo credits: Esther Leystra (2019). Nepal: Bardiya National Park. Acknowledgement I’d like to thank my supervisor Jasper Griffioen for the opportunity to hydrologically explore Bardiya. His enthusiasm for the project was inspiring and his close involvement was very motivating. My friend Ewa van Kooten introduced me to this project. Together we travelled to Nepal for three months. Thanks to her I enjoyed every single day of our time in Bardiya. She often came up with new ideas for field measurements, creative ways to fabricate field equipment or interpretations for unexpected observations. I am grateful for the Himalayan Tiger Foundation (HTF), who took the initial initiative for hydrological research in Bardiya. I very much appreciate their efforts for the conservation of the wild tiger. During the meetings in the Netherlands and around the campfire in Bardiya with the members and co of HTF, I have learned and laughed a lot. Moreover, I like to thank them for getting us in touch with the National Trust for Nature Conservation (NTNC). The staff of NTNC heartily welcomed us in Bardiya and at their office. They made us feel like a part of the NTNC-family by letting us join their festivals, dinners and campfires. -

Identification Notes &~@~-/~: ~~*~@~,~ 'PTILE
CATEGORY Identification Notes &~@~-/~: ~~*~@~,~ ‘PTILE for wildlife law enforcement ~ C.rnrn.n N.rn./s: Al@~O~, c~~~dil., ~i~.xl, Gharial PROBLEM: Skulls of Crocodilians are often imported as souvenirs. nalch (-”W 4(JI -“by ieeth ??la&ularJy+i9 GUIDE TO PRELIMINARY IDENTIFICATION OF CROCODILL4N SKULLS 1. Nasal bones separated from nasal aperture; mandibular symphysis extends to the 15th tooth. 2. Gavialis gangeticus Nasal bones entering the nasal aperture; mandibular symphysisdoes not extend beyond the8th tooth . Tomistoma schlegelii 2. Nasal bones separated from premaxillary bones; 27 -29maxi11aryteeth,25 -26mandibularteeth Nasal bones in contact with premaxillaq bo Qoco@khs acutus teeth, 18-19 mandibular teeth . Tomiitomaschlegelii 3. Fourth mandibular tooth usually fitting into a notch in the maxilla~, 16-19 maxillary teeth, 14-15 mandibular teeth . .4 Osteolaemus temaspis Fourth mandibular tooth usually fitting into a pit in the maxilla~, 14-20 maxillary teeth, 17-22 mandibular teeth . .5 4. Nasal bones do not divide nasal aperture. .. CrocodylW (12 species) Alligator m&siss@piensh Nasalboncx divide nasal aperture . Osteolaemustetraspk. 5. Nasal bones do not divide nasal aperture. .6 . Paleosuchus mgonatus Bony septum divides nasal aperture . .. Alligator (2 species) 6. Fiveteethinpremaxilla~ bone . .7 . Melanosuchus niger Four teeth in premaxillary bone. ...Paleosuchus (2species) 7. Vomerexposed on the palate . Melanosuchusniger Caiman crocodiles Vomer not exposed on palate . ...”..Caiman (2species) Illustrations from: Moo~ C. C 1921 Me&m, F. 19S1 L-.. Submitted by: Stephen D. Busack, Chief, Morphology Section, National Fish& Wildlife Forensics LabDate submitted 6/3/91 Prepared in cooperation with the National Fkh & Wdlife Forensics Laboratoy, Ashlar@ OR, USA ‘—m More on reverse side>>> IDentMcation Notes CATEGORY: REPTILE for wildlife law enforcement -- Crocodylia II CAmmom Nda Alligator, Crocodile, Caiman, Gharial REFERENCES Medem, F. -
Review Paper Status of Tiger and Its Conservation Efforts in Nepal
International Journal of Global Science Research ISSN: 2348-8344 (Online) Vol. 7, Issue 1, April 2020, pp. 1277-1283 DOI: 10.26540/ijgsr.v7.i1.2020.149 Available Online at www.ijgsr.com © Copyright 2014 | ijgsr.com | All Rights Reserved Review Paper Status of tiger and its conservation efforts in Nepal: A review Nabina Dhakal1*, Sami Shrestha2 and Jiban Shrestha3 1Agriculture and Forestry University, Rampur, Chitwan, Nepal 2Institute of Forestry, Tribhuvan University, Hariyokharka, Pokhara-15, Nepal 3Nepal Agricultural Research Council, Agriculture Botany Division, Khumaltar, Lalitpur, Nepal *Corresponding author email: [email protected] Received: 01/03/2020 Revised: 14/03/2020 Accepted: 30/03/2020 Abstract: Tiger (Panthera tigris) is one of INTRODUCTION the most endangered wildlife species in Tiger (Panthera tigris) is considered as Nepal. Currently there are 235 tigers symbol of strength, mysterious and noble counted in Nepal. The areas of habitat of beings. It is the largest cat (feline) species tigers have been extended from 4502.5 km2 with pattern of dark vertical stripes on (before 2010) to 6167.12 km2 (after 2018). reddish-orange fur with a lighter underside Five national parks (Chitwan, Bardia, along with muscular body, powerful Banke, Shuklaphata and Parsa National forelimbs, large head and a tail that is about Parks), four protection forest (Brandabhar, half the length of the body. They are Khata, Baanta and Laljhadi Protection generally different in size with Forest) and one conservation area distinguishable sexual dimorphism (Krishnasaar Conservation Area) are between males and females, with females preserving tigers in Nepal. Tigers have being smaller than males. A 2016 survey been facing extinction due to poaching, loss found out 3,890 wild tigers and 5,000 of habitat from urbanization and specimens in the United States (WWF, deforestation, and depletion of prey 2018).