Opinion of the Scientific Panel on Animal Health and Welfare on a Request from the Commission Related To
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
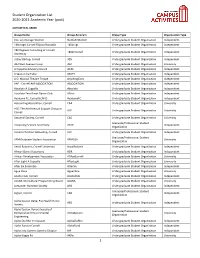
Student Organization List 2020-2021 Academic Year (Past)
Student Organization List 2020-2021 Academic Year (past) ALPHABETICAL ORDER Group Name Group Acronym Group Type Organization Type (not so) Average Women NotSoAvWomen Undergraduate Student Organization Independent 14Strings! Cornell Filipino Rondalla 14Strings Undergraduate Student Organization Independent 180 Degrees Consulting at Cornell 180dcCornell Undergraduate Student Organization Independent University 3 Day Startup, Cornell 3DS Undergraduate Student Organization Independent 302 Wait Avenue Co-op 302 Undergraduate Student Organization University A Cappella Advisory Council ACAC Undergraduate Student Organization Independent A Seat at the Table ASATT Undergraduate Student Organization Independent A.G. Musical Theatre Troupe AnythingGoes Undergraduate Student Organization Independent AAP - Cornell AAP ASSOCIATION ASSOCIATION Undergraduate Student Organization Independent Absolute A Cappella Absolute Undergraduate Student Organization Independent Absolute Zero Break Dance Club AZero Undergraduate Student Organization Independent Academy FC, Cornell (CAFC) AcademyFC Undergraduate Student Organization Independent Accounting Association, Cornell CAA Undergraduate Student Organization University ACE: The Ace/Asexual Support Group at ACE Undergraduate Student Organization University Cornell Actuarial Society, Cornell CAS Undergraduate Student Organization University Graduate/Professional Student Advancing Science And Policy ASAP Independent Organization Advent Christian Fellowship, Cornell ACF Undergraduate Student Organization Independent -

Science in the Service of Animal Welfare
Science in the Service of Animal Welfare Universities Federation for Animal Welfare Annual Report 2008-2009 Annual Report The Universities Federation for Animal Welfare The Universities Federation for Animal Welfare, founded in 1926, is an internationally recognised, independent, scientific and educational animal welfare charity concerned with promoting high standards of welfare for farm, companion, laboratory and captive wild animals, and for those animals with which we interact in the wild. It works to improve animals’ lives by: • Promoting and supporting developments in the science and technology that underpin advances in animal welfare • Promoting education in animal care and welfare • Providing information, organising meetings, and publishing books, videos, articles, technical reports and the journal Animal Welfare • Providing expert advice to government departments and other bodies and helping to draft and amend laws and guidelines • Enlisting the energies of animal keepers, scientists, veterinarians, lawyers and others who care about animals Photograph Credits Dr Cathryn Mellersh p3 courtesy of the Animal Health Trust. Broiler p7 courtesy of Louise Buckley. Sheep p9 courtesy of Bluemoondog Pictures. Elephant p9 courtesy of Dr Chris Sherwin. Zoo Outreach p10 courtesy of The Zoo Outreach Organisation. © UFAW 2009. Published by UFAW, The Old School, Brewhouse Hill, Wheathampstead, Hertfordshire AL4 8AN, UK. Tel: +44 1582 831818 Fax: +44 1582 831414 Website: www.ufaw.org.uk Email: [email protected] Printed on NAPM approved recycled paper Science in the Service of Animal Welfare 1 Letter from the Chief Executive’s Chairman Report It gives me great pleasure to Fifty years ago William report another very Russell and Rex Burch’s ‘The successful year for the Principles of Humane charity with many notable Experimental Technique’ achievements, confirmation was published. -

Bringing Animal Protection Legislation Into Line with Its Purported Purposes: a Proposal for Equality Amongst Non- Human Animals
Pace Environmental Law Review Volume 37 Issue 2 Spring 2020 Article 1 May 2020 Bringing Animal Protection Legislation Into Line With its Purported Purposes: A Proposal for Equality Amongst Non- Human Animals Jane Kotzmann Deakin University Gisela Nip Follow this and additional works at: https://digitalcommons.pace.edu/pelr Part of the Animal Law Commons, Energy and Utilities Law Commons, Environmental Law Commons, International Law Commons, and the Natural Resources Law Commons Recommended Citation Jane Kotzmann and Gisela Nip, Bringing Animal Protection Legislation Into Line With its Purported Purposes: A Proposal for Equality Amongst Non-Human Animals, 37 Pace Envtl. L. Rev. 247 (2020) Available at: https://digitalcommons.pace.edu/pelr/vol37/iss2/1 This Article is brought to you for free and open access by the School of Law at DigitalCommons@Pace. It has been accepted for inclusion in Pace Environmental Law Review by an authorized administrator of DigitalCommons@Pace. For more information, please contact [email protected]. ARTICLE Bringing Animal Protection Legislation Into Line With its Purported Purposes: A Proposal for Equality Amongst Non-Human Animals JANE KOTZMANN* & GISELA NIP† The United States has a strong history of enacting laws to pro- tect animals from the pain and suffering inflicted by humans. In- deed, the passage of the Massachusetts’ Body of Liberties in 1641 made it the first country in the world to pass such laws. Neverthe- less, contemporary animal protection laws in all jurisdictions of the United States are limited in their ability to adequately realize their primary purpose of protecting animals from unnecessary or unjus- tifiable pain and suffering. -

Veterinary Public Health
Veterinary Public Health - MPH Increasing focus on zoonotic diseases, foodborne illness, public health preparedness, antibiotic resistance, the human-animal bond, and environmental health has dramatically increased opportunities for public health veterinarians - professionals who address key issues surrounding human and animal health. Adding the MPH to your DVM degree positions you to work at the interface of human wellness and animal health, spanning agriculture and food industry concerns, emerging infectious diseases, and ecosystem health. Unique Features Curriculum Veterinary Public Health • Earn a MPH degree in the same four 42 credits MPH Program Contacts: years as your DVM. Core Curriculum (21.5 credits) • PubH 6299 - Public Health is a Team Sport: The Power www.php.umn.edu • The MPH is offered through a mix of Collaboration (1.5 cr) of online and in-person classes. Online • PubH 6020-Fundanmentals of Social and Behavioral Program Director: courses are taken during summer Science (3 cr) Larissa Minicucci, DVM, MPH terms, before and during your • PubH 6102 - Issues in Environmental and [email protected] veterinary curriculum. Attendance at Occupational Health (2 cr) 612-624-3685 the Public Health Institute, held each • PubH 6320 - Fundamentals of Epidemiology (3 cr) Program Coordinator: summer at the University of • PubH 6414 - Biostatistical Methods (3 cr) Sarah Summerbell, BS Minnesota, provides you with the • PubH 6741 - Ethics in Public Health: Professional [email protected] opportunity to earn elective credits. Practice and Policy (1 cr) 612-626-1948 The Public Health Institute is a unique • PubH 6751 - Principles of Management in Health forum for professionals from multiple Services Organizations (2 cr) disciplines to connect and immerse • PubH 7294 - Master’s Project (3 cr) Cornell Faculty Liaisons: themselves in emerging public health • PubH 7296 - Field Experience (3 cr) Alfonso Torres, DVM, MS, PhD issues. -

One Health: Veterinary Involvement and Zoonoses in Humans
Wright State University CORE Scholar Master of Public Health Program Student Publications Master of Public Health Program 2014 One Health: Veterinary Involvement and Zoonoses in Humans Jasmine Cheeks Wright State University - Main Campus Follow this and additional works at: https://corescholar.libraries.wright.edu/mph Part of the Public Health Commons Repository Citation Cheeks, J. (2014). One Health: Veterinary Involvement and Zoonoses in Humans. Wright State University, Dayton, Ohio. This Master's Culminating Experience is brought to you for free and open access by the Master of Public Health Program at CORE Scholar. It has been accepted for inclusion in Master of Public Health Program Student Publications by an authorized administrator of CORE Scholar. For more information, please contact library- [email protected]. Running Head: VETERINARY INVOLVEMENT, ZOONOSES IN HUMANS 1 One Health: Veterinary Involvement and Zoonoses in Humans An Assessment of Veterinary Professionals and Zoonotic Disease Surveillance Systems Jasmine Cheeks, MPH Wright State University Global Health Concentration VETERINARY INVOLVEMENT, ZOONOSES IN HUMANS 2 Acknowledgements Special thanks go to Dr. Nikki Rogers and Christopher Eddy for serving as Academic Reader and Chair respectively. Wright State University’s Center for Global Health and the Master of Public Health Program was instrumental in providing the educational foundation to compose and investigate this issue further. The author also thanks Daziah Merideth for her vital support throughout the composition -

Reducing Su Ering Among Invertebrates Such As Insects Policy
Reducing suering among invertebrates such as insects Policy paper Invertebrates such as insects, spiders, worms and snails may very well feel pain, and we should therefore take actions to reduce their potential suering. The large number of such invertebrate individuals and the severity of the harms that they endure mean that their potential suering would be an ethical disaster. Sentience Politics advocates that actions should be taken in several areas: Invertebrates should, when possible, not be used in re- search and teaching, and should not be used as food and feed or in the production of silk, shellac, etc. If invertebrates are to be used in these areas, we advocate actions to at least reduce their suering. In addition, attempts to prevent invertebrates from damaging crops should use the least painful methods, and research should be done to develop methods that cause less suering. Finally, policy analysis should take into account the resulting amounts of suering among all invertebrates, whether it is caused by humans or not. May Policy paper by Sentience Politics. Preferred Citation: Knutsson, S. (). Reducing suering among invertebrates such as insects. Pol- icy paper by Sentience Politics ():-. First release May . Last update May . Website: sentience-politics.org Contents Summary . Introduction . Invertebrates such as insects may very well be able to feel pain . Vast amounts of potential suering suggest that we should care about invertebrates . Actions should be taken in several areas where inverte- brates are harmed . Objections and replies . References . Author: S K, Researcher, Foundational Research Institute Project leader: T B, Director of Strategy, Sentience Politics Advisor: A M, President, Sentience Politics Advisor: B T, Researcher Lead, Foundational Research Institute Layout editor: A P Language editor: J S Reducing suering among invertebrates such as insects Summary number of scientists have argued, researchers could be re- quired to induce insensibility to pain and suering before doing potentially painful research on invertebrates. -

Minds Without Spines: Evolutionarily Inclusive Animal Ethics
Animal Sentience 2020.329: Mikhalevich & Powell on Invertebrate Minds Call for Commentary: Animal Sentience publishes Open Peer Commentary on all accepted target articles. Target articles are peer-reviewed. Commentaries are editorially reviewed. There are submitted commentaries as well as invited commentaries. Commentaries appear as soon as they have been reviewed, revised and accepted. Target article authors may respond to their commentaries individually or in a joint response to multiple commentaries. INSTRUCTIONS FOR COMMENTATORS Minds without spines: Evolutionarily inclusive animal ethics Irina Mikhalevich Department of Philosophy, Rochester Institute of Technology Russell Powell Department of Philosophy, Boston University Abstract: Invertebrate animals are frequently lumped into a single category and denied welfare protections despite their considerable cognitive, behavioral, and evolutionary diversity. Some ethical and policy inroads have been made for cephalopod molluscs and crustaceans, but the vast majority of arthropods, including the insects, remain excluded from moral consideration. We argue that this exclusion is unwarranted given the existing evidence. Anachronistic readings of evolution, which view invertebrates as lower in the scala naturae, continue to influence public policy and common morality. The assumption that small brains are unlikely to support cognition or sentience likewise persists, despite growing evidence that arthropods have converged on cognitive functions comparable to those found in vertebrates. The exclusion of invertebrates is also motivated by cognitive-affective biases that covertly influence moral judgment, as well as a flawed balancing of scientific uncertainty against moral risk. All these factors shape moral attitudes toward basal vertebrates too, but they are particularly acute in the arthropod context. Moral consistency dictates that the same standards of evidence and risk management that justify policy protections for vertebrates also support extending moral consideration to certain invertebrates. -

Veterinary Medicine and Public Health at CDC 5/15/21, 10:25 AM
Veterinary Medicine and Public Health at CDC 5/15/21, 10:25 AM December 22, 2006 / 55(SUP02);7-9 Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: [email protected]. Type 508 Accommodation and the title of the report in the subject line of e-mail. Veterinary Medicine and Public Health at CDC Lonnie J. King, DVM Office of the Director, National Center for Zoonotic, Vector-Borne, and Enteric Diseases (proposed) Corresponding author: Lonnie J. King, DVM, National Center for Zoonotic, Vector-Borne, and Enteric Diseases (proposed), CDC, 1600 Clifton Rd., N.E., MS D-76, Atlanta, GA 30333. Telephone: 404-639-7380; Fax: 404-639-7369; E-mail: [email protected]. Introduction People readily associate the role of veterinarians with private veterinary practice focused on pets and farm animals, but the true dimensions and contributions of veterinary medicine are much broader and reflect expanding societal needs and contemporary challenges to animal and human health and to the environment (1). Veterinary medicine has responsibilities in biomedical research; ecosystem management; public health; food and agricultural systems; and care of companion animals, wildlife, exotic animals, and food animals. The expanding role of veterinarians at CDC reflects an appreciation for this variety of contributions. Veterinarians' educational background in basic biomedical and clinical sciences compare with that of physicians. However, unlike their counterparts in human medicine, veterinarians must be familiar with multiple species, and their training emphasizes comparative medicine. Veterinarians are competent in preventive medicine, population health, parasitology, zoonoses, and epidemiology, which serve them well for careers in public health. -

The Welfare of Domestic Fowl and Other Captive Birds Animal Welfare
The Welfare of Domestic Fowl and Other Captive Birds Animal Welfare VOLUME 9 Series Editor Clive Phillips, Professor of Animal Welfare, Centre for Animal Welfare and Ethics, School of Veterinary Science, University of Queensland, Australia Titles published in this series: Volume 1: The Welfare of Horses Natalie Waran ISBN 1-4020-0766-3 Volume 2: The Welfare of Laboratory Animals Eila Kaliste ISBN 1-4020-2270-0 Volume 3: The Welfare of Cats Irene Rochlitz ISBN 978-1-4020-3226-4 Volume 4: The Welfare of Dogs Kevin Stafford ISBN 978-1-4020-4361-1 Volume 5: The Welfare of Cattle Jeffrey Rushen, Anne Marie de Passillé, Marina A.G. von Keyserlingk and Daniel M. Weary ISBN 978-1-4020-6557-6 Volume 6: The Welfare of Sheep Cathy M. Dwyer ISBN 978-1-4020-8552-9 Volume 7: The Welfare of Pigs Jeremy N. Marchant-Forde ISBN 978-1-4020-8908-4 Volume 8: The Welfare of Animals Clive Phillips ISBN 978-1-4020-9218-3 The Welfare of Domestic Fowl and Other Captive Birds Edited by Ian J.H. Duncan University of Guelph, Guelph, Ontario, Canada Penny Hawkins Royal Society for the Prevention of Cruelty to Animals, Southwater, UK 123 Editors Dr. Ian J.H. Duncan Dr. Penny Hawkins University of Guelph Royal Society for the Prevention of Cruelty Dept. Animal & Poultry Science to Animals Guelph ON N1G 2W1 Research Animals Dept. Canada Wilberforce Way [email protected] Horsham, W. Sussex Southwater United Kingdom RH13 9RS [email protected] ISSN 1572-7408 ISBN 978-90-481-3649-0 e-ISBN 978-90-481-3650-6 DOI 10.1007/978-90-481-3650-6 Springer Dordrecht Heidelberg London New York Library of Congress Control Number: 2009942767 © Springer Science+Business Media B.V. -

Sri Venkateswara Veterinary University, Tirupati
Volume - 15 Issue - 8 Jan-March, 2019 SRI VENKATESWARA VETERINARY UNIVERSITY, TIRUPATI Visit us at : svvu.edu.in From the Desk of Hon'ble Vice-Chancellor TIME TO RE-ORIENT OUR APPROACH Patron It gives me immense pleasure to announce that SVVU got two Dr. Y. Hari Babu mega projects of International collaboration coordinated by Royal Vice-Chancellor Veterinary College at London and the Scientific Research of Veterinary Republic of Tunisia. Chief Editor During this quarter, University has focused on the capacity building programmes for field Veterinarians, shepherds and dairy Dr. D. Sreenivasulu farmers, organization of kisan mela and breeding ram distribution Director of Extension at LRS, Palamaner, organization of special NSS camps, inaugurations of Diamond Jubilee pylon (1955- 2015), new boys hostel at CVSc, Advisors Tirupati and 10th sports, games, cultural and literary meet at CFSc., Dr. D. Srinivasa Rao Muthukur and a national conference organized by Dept. of Registrar Veterinary Parasitology of CVSc, Tirupati. The Principal Secretary, AHDD & Fishery, AP visited the campus Dr. T.S. Chandrasekhara Rao Dean, Faculty of Veterinary and reviewed the activities of University. The then Hon’ble Chief Science Minister of Andhra Pradesh, inaugurated the new spacious Veterinary Clinical Complex building with the state of art Dr. V. Padmanabha Reddy equipments to cater the needs of animal owners. The activities of Dean, Faculty of Dairy Science KVK, Lam, Guntur were remarkably appreciated by the farmers. The Dr. Y. Hari Babu, Vice-Chancellor work progress on conservation of Ongole and Punganur was Sri Venkateswara Veterinary University, Tirupati. Dr. T.V. Ramana Dean, Faculty of Fishery Science appreciated by the Principal Secretary. -

A Defense of a Sentiocentric Approach to Environmental Ethics
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 8-2012 Minding Nature: A Defense of a Sentiocentric Approach to Environmental Ethics Joel P. MacClellan University of Tennessee, Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Ethics and Political Philosophy Commons Recommended Citation MacClellan, Joel P., "Minding Nature: A Defense of a Sentiocentric Approach to Environmental Ethics. " PhD diss., University of Tennessee, 2012. https://trace.tennessee.edu/utk_graddiss/1433 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Joel P. MacClellan entitled "Minding Nature: A Defense of a Sentiocentric Approach to Environmental Ethics." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Doctor of Philosophy, with a major in Philosophy. John Nolt, Major Professor We have read this dissertation and recommend its acceptance: Jon Garthoff, David Reidy, Dan Simberloff Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) MINDING NATURE: A DEFENSE OF A SENTIOCENTRIC APPROACH TO ENVIRONMENTAL ETHICS A Dissertation Presented for the Doctor of Philosophy Degree The University of Tennessee, Knoxville Joel Patrick MacClellan August 2012 ii The sedge is wither’d from the lake, And no birds sing. -

Can Fish Really Feel Pain?
F I S H and F I S H E R I E S , 2014, 15, 97–133 Can fish really feel pain? J D Rose1, R Arlinghaus2,3, S J Cooke4*, B K Diggles5, W Sawynok6, E D Stevens7 & C D L Wynne8 1Department of Zoology and Physiology and Neuroscience Program, University of Wyoming, Department 3166, 1000 East University Avenue, Laramie, WY 80521, USA; 2Department of Biology and Ecology of Fishes, Leibniz-Institute of Freshwater Ecology and Inland Fisheries, Mu¨ggelseedamm 310, 12587, Berlin, Germany; 3Inland Fisheries Management Laboratory, Department for Crop and Animal Sciences, Faculty of Agriculture and Horticulture, Humboldt-Universitat€ zu Berlin, Berlin, Germany; 4Fish Ecology and Conservation Physiology Laboratory, Department of Biology and Institute of Environmental Science, Carleton University, 1125 Colonel By Drive, Ottawa, ON, Canada K1S 5B6; 5DigsFish Services, 32 Bowsprit Cres, Banksia Beach, QLD 4507, Australia; 6Infofish Australia, PO Box 9793, Frenchville, Qld 4701, Australia; 7Biomedical Sciences – Atlantic Veterinary College, University of Prince Edward Island, Charlottetown, PE, Canada, C1A 4P3; 8Department of Psychology, University of Florida, Box 112250, Gainesville, FL 32611, USA Abstract Correspondence: We review studies claiming that fish feel pain and find deficiencies in the methods Steven J Cooke, Fish Ecology and Conser- used for pain identification, particularly for distinguishing unconscious detection of vation Physiology injurious stimuli (nociception) from conscious pain. Results were also frequently mis- Laboratory, Depart- interpreted and not replicable, so claims that fish feel pain remain unsubstantiated. ment of Biology and Comparable problems exist in studies of invertebrates. In contrast, an extensive litera- Institute of Environ- ture involving surgeries with fishes shows normal feeding and activity immediately mental Science, Carleton University, or soon after surgery.