Population Coding and Behavioral Choice William B Kristan Jr* and Brian K Shawl
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Auditory Experience Controls the Maturation of Song Discrimination and Sexual Response in Drosophila Xiaodong Li, Hiroshi Ishimoto, Azusa Kamikouchi*
RESEARCH ARTICLE Auditory experience controls the maturation of song discrimination and sexual response in Drosophila Xiaodong Li, Hiroshi Ishimoto, Azusa Kamikouchi* Graduate School of Science, Nagoya University, Nagoya, Japan Abstract In birds and higher mammals, auditory experience during development is critical to discriminate sound patterns in adulthood. However, the neural and molecular nature of this acquired ability remains elusive. In fruit flies, acoustic perception has been thought to be innate. Here we report, surprisingly, that auditory experience of a species-specific courtship song in developing Drosophila shapes adult song perception and resultant sexual behavior. Preferences in the song-response behaviors of both males and females were tuned by social acoustic exposure during development. We examined the molecular and cellular determinants of this social acoustic learning and found that GABA signaling acting on the GABAA receptor Rdl in the pC1 neurons, the integration node for courtship stimuli, regulated auditory tuning and sexual behavior. These findings demonstrate that maturation of auditory perception in flies is unexpectedly plastic and is acquired socially, providing a model to investigate how song learning regulates mating preference in insects. DOI: https://doi.org/10.7554/eLife.34348.001 Introduction Vocal learning in infants or juvenile birds relies heavily on the early experience of the adult conspe- cific sounds. In humans, early language input is necessary to form the ability of phonetic distinction *For correspondence: and pattern detection in the phase of auditory learning (Doupe and Kuhl, 1999; Kuhl, 2004). [email protected] Because of the strong parallels between speech acquisition of humans and song learning of song- Competing interests: The birds, and the difficulties to investigate the neural mechanisms of human early auditory memory at authors declare that no cellular resolution, songbirds have been used as a predominant model in studying memory formation competing interests exist. -
A Spiking Neuron Classifier Network with a Deep Architecture Inspired By
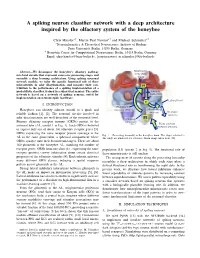
A spiking neuron classifier network with a deep architecture inspired by the olfactory system of the honeybee Chris Hausler¨ ∗†, Martin Paul Nawrot∗† and Michael Schmuker∗† ∗Neuroinformatics & Theoretical Neuroscience, Institute of Biology Freie Universitat¨ Berlin, 14195 Berlin, Germany † Bernstein Center for Computational Neuroscience Berlin, 10115 Berlin, Germany Email: [email protected], fmartin.nawrot, [email protected] Abstract—We decompose the honeybee’s olfactory pathway Mushroom into local circuits that represent successive processing stages and body (MB) calyx resemble a deep learning architecture. Using spiking neuronal network models, we infer the specific functional role of these microcircuits in odor discrimination, and measure their con- 2 tribution to the performance of a spiking implementation of a probabilistic classifier, trained in a supervised manner. The entire Kenyon network is based on a network of spiking neurons, suited for Cells (KC) implementation on neuromorphic hardware. Lateral horn 3 I. INTRODUCTION Projection Honeybees can identify odorant stimuli in a quick and neurons (PN) MB-extrinsic neurons To motor reliable fashion [1], [2]. The neuronal circuits involved in neurons odor discrimination are well described at the structural level. 1 Antennal Primary olfactory receptor neurons (ORNs) project to the lobe (AL) From odorant antennal lobe (AL, circuit 1 in Fig. 1). Each ORN is believed receptor neurons to express only one of about 160 olfactory receptor genes [3]. ORNs expressing the same receptor protein converge in the Fig. 1. Processing hierarchy in the honeybee brain. The stages relevant to AL in the same glomerulus, a spherical compartment where this study are numbered for reference. -

Behavioral Choices: How Neuronal Networks Make Decisions Dispatch
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Current Biology, Vol. 13, R140–R142, February 18, 2003, ©2003 Elsevier Science Ltd. All rights reserved. PII S0960-9822(03)00076-9 Behavioral Choices: How Neuronal Dispatch Networks Make Decisions Ronald L. Calabrese Shaw and Kristan [3] asked how the decision between shortening and swimming is made by the leech — specifically at what level the antagonism To survive, animals must constantly make behavioral between these behavioral networks occurs. What they choices. The analysis of simple, almost binary, found was somewhat surprising. At the trigger level behavioral choices in invertebrate animals with there was no antagonism: stimuli that activated short- restricted nervous systems is beginning to yield ening and swimming both activated trigger neurons. insight into how neuronal networks make such Even at the decision or command neuron level, some decisions. neurons were activated by both types of stimulus. One key neuron — cell 204 — however, was strongly inhibited by stimuli that led to shortening. The neurons Simple behavioral choices often seem binary and of the swim CPG were similarly mixed in their sequential. For example, an animal perceives some- responses. Some elements were strongly inhibited by thing novel in its environment, it chooses approach shortening stimuli and others were excited by short- over withdrawal, and sensing potential food, it chooses ening stimuli. to eat or to reject the item. Such decisions often begin What is to be made of these observations? CPG with a drive that originates in a need that is, in turn, neurons are multifunctional — there is a large body of conditioned by internal state and external stimuli. -

1P MON. PM Cortex and Subcortical Auditory Nuclei
dynamic role for the vestibular system in orientation and flight control. Laboratory studies of flight behavior under illuminated and dark conditions in both static and rotating obstacle tests were carried out while administering heavy water ͑D2O͒ to bats to impair their vestibular inputs. Eptesicus carried out complex maneuvers through both fixed arrays of wires and a rotating obstacle array using both vision and echolocation, or when guided by echolocation alone. When treated with D2O in combination with lack of visual cues, bats showed considerable decrements in performance. These data indicate that big brown bats use both vision and echolocation to provide spatial registration for head position information generated by the vestibular system. 2:55 1pAB3. Bat’s auditory system: Corticofugal feedback and plasticity. Nobuo Suga ͑Dept. of Biol., Washington Univ., One Brookings Dr., St. Louis, MO 63130͒ The auditory system of the mustached bat consists of physiologically distinct subdivisions for processing different types of biosonar information. It was found that the corticofugal ͑descending͒ auditory system plays an important role in improving and adjusting auditory signal processing. Repetitive acoustic stimulation, cortical electrical stimulation or auditory fear conditioning evokes plastic changes of the central auditory system. The changes are based upon egocentric selection evoked by focused positive feedback associated with lateral inhibition. Focal electric stimulation of the auditory cortex evokes short-term changes in the auditory 1p MON. PM cortex and subcortical auditory nuclei. An increase in a cortical acetylcholine level during the electric stimulation changes the cortical changes from short-term to long-term. There are two types of plastic changes ͑reorganizations͒: centripetal best frequency shifts for expanded reorganization of a neural frequency map and centrifugal best frequency shifts for compressed reorganization of the map. -

Fixed Action Patterns and the Central Nervous System
9.20 M.I.T. 2013 Lecture #6 Fixed Action Patterns and the Central Nervous System 1 Scott ch 2, “Controlling behavior: the role of the nervous system” 3. Give an example of a “supernormal stimulus” that acts as a releaser of a fixed action pattern in herring gull chicks. (See p 21) • See the conspicuous red-orange spot on the beak of an adult Herring gull on the next slide. Gull chicks respond to this visual stimulus with a gaping response—which elicits a feeding response from the parent. • A stronger gaping response can be elicited by a human who moves a yellow pencil painted with an orange spot. The spot plus the movement forms a “supernormal” stimulus. • Another example: Triggering the egg-rolling response from an adult gull: A larger-than-normal egg can elicit a stronger response. 2 Courtesy of Bruce Stokes on Flickr. License CC BY-NC-SA. 3 Can you give examples of supernormal stimuli for humans? 4 Supernormal stimuli for humans: • Foods: Sweet in taste, high in fats (Beware of restaurants!) • Stimuli of sexual attraction: The “poster effect” in advertizing • Enhancements of male appearance – Shoulder width, exagerated – Penis prominence enhanced: Sheath in tribal dress, cowl in medieval constumes • Enhancements of female appearance: – Waist-to-hip ratio enhancements: Girdle, bustle – Breast prominence increased – Lip color, size enhanced (How? For what purpose?) – Shoulder size: But what is the purpose of shoulder pads in women’s dress? 5 Scott ch 2, “Controlling behavior: the role of the nervous system” 4. Define: Primary sensory neuron, secondary sensory neuron, motor neuron, interneuron (neuron of the great intermediate net). -

Of the Lateral Giant Escape Neurons in Crayfish Sensory Activation And
Sensory Activation and Receptive Field Organization of the Lateral Giant Escape Neurons in Crayfish Yen-Chyi Liu and Jens Herberholz J Neurophysiol 104:675-684, 2010. First published 26 May 2010; doi:10.1152/jn.00391.2010 You might find this additional info useful... This article cites 57 articles, 31 of which can be accessed free at: http://jn.physiology.org/content/104/2/675.full.html#ref-list-1 Updated information and services including high resolution figures, can be found at: http://jn.physiology.org/content/104/2/675.full.html Additional material and information about Journal of Neurophysiology can be found at: http://www.the-aps.org/publications/jn This infomation is current as of February 10, 2012. Downloaded from jn.physiology.org on February 10, 2012 Journal of Neurophysiology publishes original articles on the function of the nervous system. It is published 12 times a year (monthly) by the American Physiological Society, 9650 Rockville Pike, Bethesda MD 20814-3991. Copyright © 2010 by the American Physiological Society. ISSN: 0022-3077, ESSN: 1522-1598. Visit our website at http://www.the-aps.org/. J Neurophysiol 104: 675–684, 2010. First published May 26, 2010; doi:10.1152/jn.00391.2010. Sensory Activation and Receptive Field Organization of the Lateral Giant Escape Neurons in Crayfish Yen-Chyi Liu1 and Jens Herberholz1,2 1Department of Psychology, 2Neuroscience and Cognitive Science Program, University of Maryland, College Park, Maryland Submitted 28 April 2010; accepted in final form 26 May 2010 Liu YC, Herberholz J. Sensory activation and receptive field 1999; Herberholz 2007; Krasne and Edwards 2002a; Wine and organization of the lateral giant escape neurons in crayfish. -

Portia Perceptions: the Umwelt of an Araneophagic Jumping Spider
Portia Perceptions: The Umwelt of an Araneophagic Jumping 1 Spider Duane P. Harland and Robert R. Jackson The Personality of Portia Spiders are traditionally portrayed as simple, instinct-driven animals (Savory, 1928; Drees, 1952; Bristowe, 1958). Small brain size is perhaps the most compelling reason for expecting so little flexibility from our eight-legged neighbors. Fitting comfortably on the head of a pin, a spider brain seems to vanish into insignificance. Common sense tells us that compared with large-brained mammals, spiders have so little to work with that they must be restricted to a circumscribed set of rigid behaviors, flexibility being a luxury afforded only to those with much larger central nervous systems. In this chapter we review recent findings on an unusual group of spiders that seem to be arachnid enigmas. In a number of ways the behavior of the araneophagic jumping spiders is more comparable to that of birds and mammals than conventional wisdom would lead us to expect of an arthropod. The term araneophagic refers to these spiders’ preference for other spiders as prey, and jumping spider is the common English name for members of the family Saltici- dae. Although both their common and the scientific Latin names acknowledge their jumping behavior, it is really their unique, complex eyes that set this family of spiders apart from all others. Among spiders (many of which have very poor vision), salticids have eyes that are by far the most specialized for resolving fine spatial detail. We focus here on the most extensively studied genus, Portia. Before we discuss the interrelationship between the salticids’ uniquely acute vision, their predatory strategies, and their apparent cognitive abilities, we need to offer some sense of what kind of animal a jumping spider is; to do this, we attempt to offer some insight into what we might call Portia’s personality. -

Krogh's Principle
Introduction to Neuroscience: Behavioral Neuroscience Neuroethology, Comparative Neuroscience, Natural Neuroscience Nachum Ulanovsky Department of Neurobiology, Weizmann Institute of Science 2017-2018, 2nd semester Principles of Neuroethology Neuroethology seeks to understand the mechanisms by which the Neurobiology central nervous system controls the Neuroethology Ethology natural behavior of animals. • Focus on Natural behaviors: Choosing to study a well-defined and reproducible yet natural behavior (either Innate or Learned behavior) • Need to study thoroughly the animal’s behavior, including in the field: Neuroethology starts with a good understanding of Ethology. • If you study the animals in the lab, you need to keep them in conditions as natural as possible, to avoid the occurrence of unnatural behaviors. • Krogh’s principle 1 Krogh’s principle August Krogh Nobel prize 1920 “For such a large number of problems there will be some animal of choice or a few such animals on which it can be most conveniently studied. Many years ago when my teacher, Christian Bohr, was interested in the respiratory mechanism of the lung and devised the method of studying the exchange through each lung separately, he found that a certain kind of tortoise possessed a trachea dividing into the main bronchi high up in the neck, and we used to say as a laboratory joke that this animal had been created expressly for the purposes of respiration physiology. I have no doubt that there is quite a number of animals which are similarly "created" for special physiological -

Lateral Inhibition Effects Demonstrate That the Maturational State of Neurons That Then Inhibit Their Neighbors
522 Lateral Inhibition effects demonstrate that the maturational state of neurons that then inhibit their neighbors. When a the learner’s brain is crucial for the attainment of stimulus (such as a bar of light or any other stim- a language system. ulus) excites a number of neurons in the network Several questions remain open about the mecha- (in this case neurons 4e, 5e, 6e, and 7e), the effect nisms underlying language learning. One issue is of inhibition is to suppress the neurons just out- whether specific aspects of language acquisition side the edge of the bar (3e and 8e) because those should be attributed to language-specific versus neurons are inhibited but not excited. Further, general-purpose learning mechanisms. Another issue because the neurons just inside the edges of the is whether children’s native language can affect the bar (4e and 7e) are excited by light and only inhib- way they think, and whether language is necessary ited by one neighbor, they are especially active. or helpful for the development of human concepts. This leads to perceptual contrast enhancement at borders. Further research showed that lateral inhi- Anna Papafragou bition also applied to overlapping stimuli, and that its strength fell off with distance between the See also Aphasias; Audition: Cognitive Influences; Context Effects in Perception; Speech Perception; Top- interacting stimuli. Down and Bottom-Up Processing; Word Recognition Haldan Keffer Hartline won the Nobel Prize in 1967 for discovering lateral inhibition and its neu- ral correlates. The first inhibitory circuit in the Further Readings nervous system was found in the horseshoe crab (Limulus polyphemus). -

Command Neurons Are Often Defined As Neurons Which, When Stimulated by the Experimenter, Evoke Some Behavioral Response
THE BEHAVIORAL AND BRAIN SCIENCES (1978), 1,3-39 Printed in the United States of America The command neuron concept Irving Kupfermann Department of Psychiatry and Division of Neurobiologyand Behavior, College of Physicians and Surgeons of Columbia University, New York, N Y 10032 Klaudiusz R. Weiss Department of Psychiatry and Division of Neurobiology and Behavior, College of Physicians and Surgeons of Columbia University, New York, N Y 10032 Abstract: The notion of the command cell has been highly influential in invertebrate neurobiology, and related notions have been increasingly used in research on the vertebrate nervous system. The term "command neuron" implies that the neuron has some critical function in the generation of a normally occurring behavior. Nevertheless, most authors either explicitly or implicitly use a strictly operational definition, independent of considerations of normal behavioral function. That is, command neurons are often defined as neurons which, when stimulated by the experimenter, evoke some behavioral response. Even when utilizing such an operational definition, investigators frequently differ on what they consider to be the exact characteristics that a neuron must have (or not have) to be considered a command cell. A few authors appear to treat command neurons in relation to normal function, but a precise be- haviorally relevant definition has not been specified. Because of the ambiguity in the definition of command neurons, the term can refer to a wide variety of neurons which may play divergent behavioral roles. In some ways the attempt to label a cell as a command neuron may interfere with the process of discovering the complex causal determinants of behavior. -

Lecture 5 Study Questions: Ethology of Geese; Fixed Action Patterns And
9.20 Class #5 Study questions: 1. Yawning is a human “fixed action pattern” (FAP). Name three other FAPs shown by humans. Try not to name reflexes, but rather, innate patterns of behavior that have a motivational component (see next question). 2. Unlike Graham Scott, many ethologists distinguish FAPs from reflexes. How do you think these types of actions can be distinguished? Give examples. (Scott uses “reflex” to mean automatic and at least initially unlearned.) 3. Give an example of a “supernormal stimulus” that acts as a releaser of a fixed action pattern in herring gull chicks. (See p 21) 4. Define: Primary sensory neuron, secondary sensory neuron, motor neuron, interneuron (neuron of the great intermediate net). [This textbook is not as clear as I would like in discussing the nervous system. Do not depend on this book for neuroscience information. The terms will be defined in class.] 5. What are the major specializations of nerve cells, compared with other cells of the body? 6. How can a “wandering spider” catch its prey without using a web, by a kind of touch sensitivity that does not involve direct contact? 7. What features of a moving visual stimulus are detected by the visual system of a toad in the triggering of prey-catching behavior? Describe a prey-catching action of a toad or a frog. 8. Where in the central nervous system of a toad could an electrical stimulus elicit a prey-catching fixed action pattern? What would change if the electrode were moved a short distance parallel to the brain surface? 9. -

Sensory Receptors A17 (1)
SENSORY RECEPTORS A17 (1) Sensory Receptors Last updated: April 20, 2019 Sensory receptors - transducers that convert various forms of energy in environment into action potentials in neurons. sensory receptors may be: a) neurons (distal tip of peripheral axon of sensory neuron) – e.g. in skin receptors. b) specialized cells (that release neurotransmitter and generate action potentials in neurons) – e.g. in complex sense organs (vision, hearing, equilibrium, taste). sensory receptor is often associated with nonneural cells that surround it, forming SENSE ORGAN. to stimulate receptor, stimulus must first pass through intervening tissues (stimulus accession). each receptor is adapted to respond to one particular form of energy at much lower threshold than other receptors respond to this form of energy. adequate (s. appropriate) stimulus - form of energy to which receptor is most sensitive; receptors also can respond to other energy forms, but at much higher thresholds (e.g. adequate stimulus for eye is light; eyeball rubbing will stimulate rods and cones to produce light sensation, but threshold is much higher than in skin pressure receptors). when information about stimulus reaches CNS, it produces: a) reflex response b) conscious sensation c) behavior alteration SENSORY MODALITIES Sensory Modality Receptor Sense Organ CONSCIOUS SENSATIONS Vision Rods & cones Eye Hearing Hair cells Ear (organ of Corti) Smell Olfactory neurons Olfactory mucous membrane Taste Taste receptor cells Taste bud Rotational acceleration Hair cells Ear (semicircular