Disclaimer COVID-19 Evidence Support Team EVIDENCE SEARCH
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Recommendations of the Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP)
Morbidity and Mortality Weekly Report Recommendations and Reports February 8, 2002 / Vol. 51 / No. RR-2 General Recommendations on Immunization Recommendations of the Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP) INSIDE: Continuing Education Examination Centers for Disease Control and Prevention SAFER • HEALTHIER • PEOPLETM MMWR CONTENTS The MMWR series of publications is published by the Introduction ......................................................................... 1 Epidemiology Program Office, Centers for Disease Timing and Spacing of Immunobiologics .............................. 2 General Principles for Vaccine Scheduling ......................... 2 Control and Prevention (CDC), U.S. Department of Spacing of Multiple Doses of the Same Antigen ................ 2 Health and Human Services, Atlanta, GA 30333. Simultaneous Administration ............................................ 4 Nonsimultaneous Administration ...................................... 5 Spacing of Antibody-Containing Products and Vaccines ..... 6 SUGGESTED CITATION Interchangeability of Vaccines from Different Manufacturers 8 Centers for Disease Control and Prevention. General Lapsed Vaccination Schedule ............................................ 8 recommendations on immunization: recom- Unknown or Uncertain Vaccination Status ......................... 8 mendations of the Advisory Committee on Contraindications and Precautions ....................................... 8 Immunization Practices and the -

CIOMS Guide to Vaccine Safety Communication
2018 CIOMS Guide to Vaccine Safety Communication Report by Topic Group 3 of the CIOMS Working Group on Vaccine Safety Council for International Organizations of CIOMS Guide to Vaccine Safety Communication CIOMS Guide to Vaccine Medical Sciences (CIOMS) Geneva, Switzerland 2018 CIOMS CIOMS Guide to Vaccine Safety Communication Report by Topic Group 3 of the CIOMS Working Group on Vaccine Safety Council for International Organizations of Medical Sciences (CIOMS) Geneva,Geneva Switzerland 2014 2018 Copyright © 2018 by the Council for International Organizations of Medical Sciences (CIOMS) ISBN: 978-92-9036091-9 All rights reserved. CIOMS publications may be obtained directly from CIOMS using its website e-shop module at https://cioms.ch/shop/. Further information can be obtained from CIOMS P.O. Box 2100, CH-1211 Geneva 2, Switzerland, tel.: +41 22 791 6497, www.cioms.ch, e-mail: [email protected]. CIOMS publications are also available through the World Health Organization, WHO Press, 20 Avenue Appia, CH-1211 Geneva 27, Switzerland. Citations: CIOMS Guide to vaccine safety communication. Report by topic group 3 of the CIOMS Working Group on Vaccine Safety. Geneva, Switzerland: Council for International Organizations of Medical Sciences (CIOMS), 2018. Note on style: This publication uses the World Health Organization’s WHO style guide, 2nd Edition, 2013 (WHO/ KMS/WHP/13.1) wherever possible for spelling, punctuation, terminology and formatting which combines British and American English conventions. Disclaimer: The authors alone are responsible for the views expressed in this publication and those views do not necessarily represent the decisions, policies or views of their respective institutions or companies. -

Top 10 Stories from the June 2021 AMA Special Meeting
Top 10 stories from the June 2021 AMA Special Meeting JUN 17, 2021 Kevin B. O'Reilly News Editor Nearly 700 physicians, residents and medical students gathered for the June 2021 AMA Special Meeting of the AMA House of Delegates (HOD) to consider a wide array of proposals to help fulfill the AMA's core mission of promoting medicine and improving public health. As they have done since the global pandemic was declared last year, the delegates met virtually. Pandemic heroism sets path for work ahead “This is a consequential time in American history, and in the history of medicine,” new AMA President Gerald E. Harmon, MD, said in his inaugural address. The nation is “at war against seemingly formidable adversaries: the COVID-19 pandemic, which has led to the deaths of millions worldwide, and hundreds of thousands here at home, prolonged isolation and its effects on emotional and behavioral health, political and racial tension, and the immense battle to rid our health system—and society—of health disparities and racism.” Watch or read Dr. Harmon’s speech. Susan R. Bailey, MD, now the AMA’s immediate past president, told delegates that “no one has shouldered more in this pandemic than our courageous colleagues on the front lines—brave men and women from every state who have gone above and beyond in service to their patients and communities. You will remain in our hearts and in our thoughts long after this pandemic is over.” Watch or read Dr. Bailey’s speech. Executive Vice President and CEO James L. Madara, MD, detailed the role “a more nimble, focused” AMA played in supporting physicians during a once-in-a-generation public health crisis and how that response can be channeled to advance health equity. -

COVID-19 Vaccination Programme: Information for Healthcare Practitioners
COVID-19 vaccination programme Information for healthcare practitioners Republished 6 August 2021 Version 3.10 1 COVID-19 vaccination programme: Information for healthcare practitioners Document information This document was originally published provisionally, ahead of authorisation of any COVID-19 vaccine in the UK, to provide information to those involved in the COVID-19 national vaccination programme before it began in December 2020. Following authorisation for temporary supply by the UK Department of Health and Social Care and the Medicines and Healthcare products Regulatory Agency being given to the COVID-19 Vaccine Pfizer BioNTech on 2 December 2020, the COVID-19 Vaccine AstraZeneca on 30 December 2020 and the COVID-19 Vaccine Moderna on 8 January 2021, this document has been updated to provide specific information about the storage and preparation of these vaccines. Information about any other COVID-19 vaccines which are given regulatory approval will be added when this occurs. The information in this document was correct at time of publication. As COVID-19 is an evolving disease, much is still being learned about both the disease and the vaccines which have been developed to prevent it. For this reason, some information may change. Updates will be made to this document as new information becomes available. Please use the online version to ensure you are accessing the latest version. 2 COVID-19 vaccination programme: Information for healthcare practitioners Document revision information Version Details Date number 1.0 Document created 27 November 2020 2.0 Vaccine specific information about the COVID-19 mRNA 4 Vaccine BNT162b2 (Pfizer BioNTech) added December 2020 2.1 1. -
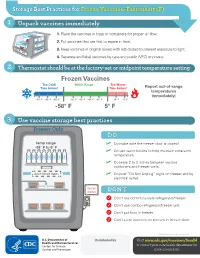
Storage Best Practices for Frozen Vaccines-Fahrenheit
Storage Best Practices for Frozen Vaccines–Fahrenheit (F) 1 Unpack vaccines immediately 1. Place the vaccines in trays or containers for proper air flow. 2. Put vaccines that are first to expire in front. HEP A - VFC 3. Keep vaccines in original boxes with lids closed to prevent exposure to light. 4. Separate and label vaccines by type and public (VFC) or private. 2 Thermostat should be at the factory-set or midpoint temperature setting Frozen Vaccines Too Cold! Within Range Too Warm! Take Action! Take Action! Report out-of-range temperatures immediately! -70° F -65° F -60° F -50° F -45° F -40° F -35° F 10° F 15° F -58° F 5° F 3 Use vaccine storage best practices Freezer Only DO temp range ✓ Do make sure the freezer door is closed! -58° F to 5° F ✓ Do use water bottles to help maintain consistent temperature. ✓ Do leave 2 to 3 inches between vaccine containers and freezer walls. don’t block vents ✓ Do post “Do Not Unplug” signs on freezer and by electrical outlet. do not unplug DON’T Don’t use dormitory-style refrigerator/freezer. Don’t use combo refrigerator/freezer unit. Don’t put food in freezer. Don’t store vaccines on shelves in freezer door. CS243541-D Revision December 2020 Distributed by Visit www.cdc.gov/vaccines/SandH or contact your state health department for more information. Test Your Knowledge 1 Which of the following units is the best for storing frozen vaccines? Freezer Freezer Freezer Freezer A. Full-size B. Full-size C. Stand-alone D. -

Frequently Asked Questions About COVID-19
doh.sd.gov/covid/ Frequently Asked Questions About COVID-19 VACCINE SAFETY Why should I get vaccinated for COVID-19? COVID-19 can cause serious illness or even death. There’s no way to know how COVID-19 will affect you. And if you get sick, you could spread the disease to friends, family, and others around you, putting their lives at risk. Getting a COVID-19 vaccine greatly reduces the risk that you’ll develop COVID- 19. The vaccines prevent nearly 100% of hospitalizations and deaths due to COVID-19. Are the COVID-19 vaccines safe? Yes. The COVID-19 vaccines available in the United States meet the FDA’s rigorous standards for safety and effectiveness. Tens of millions of people in the United States have received COVID-19 vaccines, and all COVID vaccines will continue to be monitored for safety. Serious health effects from vaccines are very rare. It’s highly unlikely that COVID-19 vaccines will cause long-term health problems. Also, there is no evidence at all that they will cause infertility or cancer. Your risk for serious health problems is much lower from the vaccine than your risk if you’re unvaccinated and get COVID-19. COVID-19 can leave you with heart and lung damage and other conditions that require long-term treatment. Vaccines are much safer paths to immunity than the disease itself. How can COVID-19 vaccines be safe since they were developed so fast? Safe COVID-19 vaccines were developed quickly through the use of a century of vaccine experience; technology that was new to vaccines but had been studied for two decades; a coronavirus vaccine already in development at the National Institutes of Health; and tens of thousands of volunteers for clinical trials that enabled rapid accumulation of data on safety and effectiveness. -

CDC Vaccine Storage and Handling Guide
Table of Contents General Information Vaccine Storage and Handling Best Practices 5 Selected Biologicals Diphtheria Toxoid-, Tetanus Toxoid- and acellular Pertussis-Containing Vaccines DTaP: DAPTACEL, Infanrix, Tripedia 11 DTaP-IPV: KINRIX 11 DTaP-HepB-IPV: Pediarix 11 DTaP-IPV/Hib: Pentacel 11 Haemophilus influenzae type b-Containing Vaccines Hib: ActHIB, Hiberix, PedvaxHIB 15 Hib-HepB: Comvax 15 DTaP-IPV/Hib: Pentacel 11 Hepatitis-Containing Vaccines HepA: Havrix, VAQTA 19 HepB: Engerix-B, Recombivax HB 19 HepA-HepB: Twinrix 19 Vaccine Storage and Handling Guide and Storage Vaccine National Center for Immunization and Respiratory Disease DTaP-HepB-IPV: Pediarix 11 Hib-HepB: Comvax 15 Human Papillomavirus Vaccines HPV2: Cervarix 23 HPV4: Gardasil 23 Vaccine Storage and Handling Guide —————————————————————————————— Page 2 Table of Contents Influenza Vaccines LAIV: FluMist 27 TIV: Afluria, Fluarix, FluLaval, Fluvirin, Fluzone, Fluzone High-Dose, Fluzone Intradermal 29 Measles-, Mumps- and Rubella-Containing Vaccine MMR: M-M-RII 33 MMRV: ProQuad 69 Meningococcal Vaccines MCV4: Menactra, Menveo 37 MPSV4: Menomune 41 Pneumococcal Vaccines PCV13: Prevnar 13 45 PPSV23: Pneumovax 23 45 Poliovirus-Containing Vaccine IPV: IPOL 49 DTaP-HepB-IPV: Pediarix 11 DTaP-IPV: KINRIX 11 Vaccine Storage and Handling Guide and Storage Vaccine National Center for Immunization and Respiratory Disease DTaP-IPV/Hib: Pentacel 11 Rotavirus Vaccines RV1: ROTARIX 53 RV5: RotaTeq 53 Tetanus Toxoid Vaccine TT: Tetanus Toxoid 57 Vaccine Storage and Handling Guide —————————————————————————————— -

Prospecting for an HIV Vaccine D
Brett-Major et al. Tropical Diseases, Travel Medicine and Vaccines (2017) 3:6 DOI 10.1186/s40794-017-0050-4 REVIEW Open Access Prospecting for an HIV vaccine D. M. Brett-Major1,2*, T. A. Crowell1,2 and N. L. Michael1 Abstract Human immunodeficiency virus (HIV) sets several challenges for the development of a preventative HIV vaccine. Predictable, protective natural immunity against HIV does not occur and so unlike most other diseases for which vaccines exist, there are few guideposts from natural infection. Nonetheless, six vaccine efficacy trials have occurred. One in particular, the Thai trial called RV144, showed partial protective efficacy and potential ways ahead to a better vaccine approach. This coupled with other lessons from studies of acute infections as well as an increasingly complex knowledge of HIV-related vaccine immunology bring hope that a vaccine solution might be reached for this pervasive and deadly pandemic. Keywords: Human immunodeficiency virus vaccine protective efficacy immunology review Background Why not already Human immunodeficiency virus (HIV) disease remains A reasonable person new to the global conversation one of the greatest threats to global public health. about HIV might ask, if an HIV vaccine is so critical and According to the World Health Organization (WHO), in the pandemic known for three decades, why do we not 2014 over one million people died from HIV, nearly already have an HIV vaccine? There is no simple answer thirty-seven million people had chronic infection and to this question, though an easy one is that people do two million people newly acquired infections [1]. Of not develop natural, protective immunity to HIV infec- those persons known to be HIV infected, only 35% tion and disease. -

The Model of “Informed Refusal” for Vaccination: How to Fight Against Anti-Vaccinationist Misinformation Without Disregarding the Principle of Self-Determination
Communication The Model of “Informed Refusal” for Vaccination: How to Fight against Anti-Vaccinationist Misinformation without Disregarding the Principle of Self-Determination Stefano D’Errico 1, Emanuela Turillazzi 2, Martina Zanon 1, Rocco Valerio Viola 3, Paola Frati 3,4 and Vittorio Fineschi 3,4,* 1 Department of Surgery, Medicine and Health, University of Trieste, 34149 Trieste, Italy; [email protected] (S.D.); [email protected] (M.Z.) 2 Department of Surgical Pathology, Medical, Molecular and Critical Area, Institute of Legal Medicine, University of Pisa, 56126 Pisa, Italy; [email protected] 3 Department of Anatomical, Histological, Forensic and Orthopaedic Sciences, Sapienza University of Rome, Viale Regina Elena 336, 00161 Rome, Italy; [email protected] (R.V.V.); [email protected] (P.F.) 4 IRCCS (Istituto di Ricerca e Cura a Carattere Scientifico) Neuromed Mediterranean Neurological Institute, Via Atinense 18, 86077 Pozzilli, Italy * Correspondence: [email protected]; Tel.: +39 06 49912722 Abstract: Vaccines are arguably a public health success story as well as an incredibly cost-effective medical resource. Despite this, worldwide concerns about their safety are growing, with the risk of Citation: D’Errico, S.; Turillazzi, E.; increased morbidity and mortality in vaccine-preventable diseases because of vaccine refusal. The Zanon, M.; Viola, R.V.; Frati, P.; global political trend in developed countries is to increasingly reduce mandates and the compulsory Fineschi, V. The Model of “Informed nature of vaccination programs. This is due to strong opposition from anti-vaccination movements Refusal” For Vaccination: How to and groups. While these have existed since the beginnings of vaccinology, they have recently gained Fight Against Anti-Vaccinationist a strong foothold through massive exploitation of the media and especially the internet. -

An Overview of COVID Vaccine Clinical Trial Results & Some Challenges
An overview of COVID vaccine clinical trial results & some challenges DCVMN Webinar December 8th, 2020 Access to COVID-19 tools ACCESSACCESS TO TOCOVID-19 COVID-19 TOOLS TOOLS (ACT) (ACT) ACCELERATOR ACCELERATOR (ACT) accelerator A GlobalA GlobalCollaboration Collaboration to Accelerate tothe AccelerateDevelopment, the Production Development, and Equitable Production Access to New and Equitable AccessCOVID-19 to New diagnostics, COVID-19 therapeutics diagnostics, and vaccines therapeutics and vaccines VACCINES DIAGNOSTICS THERAPEUTICS (COVAX) Development & Manufacturing Led by CEPI, with industry Procurement and delivery at scale Led by Gavi Policy and allocation Led by WHO Key players SOURCE: (ACT) ACCELERATOR Commitment and Call to Action 24th April 2020 ACT-A / COVAX governance COVAX COORDINATION MEETING CEPI Board Co-Chair: Jane Halton Co-Chair: Dr. Ngozi Gavi Board Workstream leads + DCVMN and IFPMA-selected Reps As needed – R&D&M Chair; COVAX IPG Chair Development & Manufacturing Procurement and delivery Policy and allocation (COVAX) at scale Led by (with industry) Led by Led by R&D&M Investment Committee COVAX Independent Product Group Technical Review Group Portfolio Group Vaccine Teams SWAT teams RAG 3 COVAX SWAT teams are being set up as a joint platform to accelerate COVID- 19 Vaccine development and manufacturing by addressing common challenges together Timely and targeted Multilateral Knowledge-based Resource-efficient Addresses specific cross- Establishes a dialogue Identifies and collates Coordinates between developer technical and global joint effort most relevant materials different organizations/ challenges as they are across different COVID-19 and insights across the initiatives to limit raised and/or identified vaccines organizations broader COVID-19 duplications and ensure on an ongoing basis (incl. -

Road to Recovery: Recovering from Post-Acute COVID-19 (Long COVID)
Road to Recovery: Recovering from post-acute COVID-19 (long COVID) Information and Advice Name: _____________________________ Symptoms reported by patients with post-acute (long) COVID-19 2 Recovery from post-acute COVID-19 (long COVID), October 2020 What is COVID-19? Covid-19 is an infectious virus that mainly affects the lungs. It is transmitted through droplets created from sneezing and coughing. The virus enters the body via the nose, mouth and eyes. What is post-acute COVID-19 (long COVID)? It is the term for someone who has not recovered for several weeks or months following the start of symptoms that were suggestive of COVID, whether they were tested for COVID-19 or not. We do not yet know why some people’s recovery takes much longer than others. What are the symptoms of post-acute COVID-19 (long COVID)? The most commonly reported symptoms are: • Fatigue • Muscle, body aches • Difficulty breathing • As well as the physical symptoms listed, it is very common to experience feelings of anxiety and low mood. Controlling shortness of breath Relieving shortness of breath People who have had a respiratory illness can often feel short of breath (SOB) afterwards. Often daily tasks such as walking, getting dressed or doing chores around the house can cause this breathlessness. Feeling like you can’t catch your breath can make you panic or feel frightened. Learning to control these feelings of breathlessness is a skill that will help you to be less troubled by this and enable you to do more. When you are feeling breathless, do not panic. -

Effectiveness of Pfizer-Biontech and Moderna Vaccines Against COVID-19 Among Hospitalized Adults Aged ≥65 Years — United States, January–March 2021
Morbidity and Mortality Weekly Report Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Among Hospitalized Adults Aged ≥65 Years — United States, January–March 2021 Mark W. Tenforde, MD, PhD1; Samantha M. Olson, MPH1; Wesley H. Self, MD2; H. Keipp Talbot, MD2; Christopher J. Lindsell, PhD2; Jay S. Steingrub, MD3; Nathan I. Shapiro, MD4; Adit A. Ginde, MD5; David J. Douin, MD5; Matthew E. Prekker, MD6; Samuel M. Brown, MD7; Ithan D. Peltan, MD7; Michelle N. Gong, MD8; Amira Mohamed, MD8; Akram Khan, MD9; Matthew C. Exline, MD10; D. Clark Files, MD11; Kevin W. Gibbs, MD11; William B. Stubblefield, MD2; Jonathan D. Casey, MD2; Todd W. Rice, MD2; Carlos G. Grijalva, MD2; David N. Hager, MD, PhD12; Arber Shehu, MD12; Nida Qadir, MD13; Steven Y. Chang, MD, PhD13; Jennifer G. Wilson, MD14; Manjusha Gaglani, MBBS15,16; Kempapura Murthy, MPH15; Nicole Calhoun, LMSW, MPA15; Arnold S. Monto, MD17; Emily T. Martin, PhD17; Anurag Malani, MD18; Richard K. Zimmerman, MD19; Fernanda P. Silveira, MD19; Donald B. Middleton, MD19; Yuwei Zhu, MD2; Dayna Wyatt2; Meagan Stephenson, MPH1; Adrienne Baughman2; Kelsey N. Womack, PhD2; Kimberly W. Hart2; Miwako Kobayashi, MD1; Jennifer R. Verani, MD1; Manish M. Patel, MD1; IVY Network; HAIVEN Investigators On April 28, 2021, this report was posted as an MMWR Early ≥65 years. Vaccination is a critical tool for reducing severe Release on the MMWR website (https://www.cdc.gov/mmwr). COVID-19 in groups at high risk. Adults aged ≥65 years are at increased risk for severe outcomes Randomized clinical trials of vaccines that have received an from COVID-19 and were identified as a priority group to EUA in the United States showed efficacy of 94%–95% in receive the first COVID-19 vaccines approved for use under preventing COVID-19–associated illness (4,5).§ However, an Emergency Use Authorization (EUA) in the United States hospitalization is a rare outcome among patients with (1–3).