Monotremes (Prototheria)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Marsupials As Models for Research
CSIRO PUBLISHING Introduction www.publish.csiro.au/journals/ajz Australian Journal of Zoology, 2006, 54, 137–138 Marsupials as models for research Lynne SelwoodA,B and Graeme CoulsonA ADepartment of Zoology, The University of Melbourne, Vic. 3010, Australia. BCorresponding author. Email: [email protected] Marsupials are worth studying for their intrinsic value alone. rather than by trying to determine what happens when the They are one of the three major extant mammal types, conceptuses are implanted in the uterus, as in the mouse. The Prototheria (monotremes), Metatheria (marsupials) and Renfree and Shaw group has skilfully exploited this in the Eutheria, and have provided important information about the tammar wallaby, and provided further experimental advan- evolution of mammals. They represent the major mammalian tages by developing techniques for gonad sex reversal and group on the Australian continent, and their study makes an female reproductive tract sex reversal in the neonates important contribution to our natural heritage. Such studies (Renfree et al. 2006). Using marsupials, the development of are necessary in order to stem the further loss of marsupial the scrotum, mammary glands, pouch and processus vagi- diversity due to extinction of species. In addition, the study nalis are shown to be sexually dimorphic before the testis of marsupial species has provided new insights into old prob- differentiates, and hence are independent of testicular hor- lems, because of their value as models to study a variety of mones. These studies have been extended into the molecular totally different fields. level. The study of marsupials can be seen as an example of the Studies on life history strategies of Antechinus showed importance of basic research. -

Miocene Mammal Reveals a Mesozoic Ghost Lineage on Insular New Zealand, Southwest Pacific
Miocene mammal reveals a Mesozoic ghost lineage on insular New Zealand, southwest Pacific Trevor H. Worthy*†, Alan J. D. Tennyson‡, Michael Archer§, Anne M. Musser¶, Suzanne J. Hand§, Craig Jonesʈ, Barry J. Douglas**, James A. McNamara††, and Robin M. D. Beck§ *School of Earth and Environmental Sciences, Darling Building DP 418, Adelaide University, North Terrace, Adelaide 5005, South Australia, Australia; ‡Museum of New Zealand Te Papa Tongarewa, P.O. Box 467, Wellington 6015, New Zealand; §School of Biological, Earth and Environmental Sciences, University of New South Wales, New South Wales 2052, Australia; ¶Australian Museum, 6-8 College Street, Sydney, New South Wales 2010, Australia; ʈInstitute of Geological and Nuclear Sciences, P.O. Box 30368, Lower Hutt 5040, New Zealand; **Douglas Geological Consultants, 14 Jubilee Street, Dunedin 9011, New Zealand; and ††South Australian Museum, Adelaide, South Australia 5000, Australia Edited by James P. Kennett, University of California, Santa Barbara, CA, and approved October 11, 2006 (sent for review July 8, 2006) New Zealand (NZ) has long been upheld as the archetypical Ma) dinosaur material (13) and isolated moa bones from marine example of a land where the biota evolved without nonvolant sediments up to 2.5 Ma (1, 14), the terrestrial record older than terrestrial mammals. Their absence before human arrival is mys- 1 Ma is extremely limited. Until now, there has been no direct terious, because NZ was still attached to East Antarctica in the Early evidence for the pre-Pleistocene presence in NZ of any of its Cretaceous when a variety of terrestrial mammals occupied the endemic vertebrate lineages, particularly any group of terrestrial adjacent Australian portion of Gondwana. -

Versión Disponible En PDF
nicolás r chimento, Federico L agnolin y Fernando e novas Museo Argentino de Ciencias Naturales Bernardino Rivadavia Necrolestes un mamífero patagónico que sobrevivió a la extinción de los dinosaurios Un descubrimiento patagónico desembocadura del río Santa Cruz, descubrieron esque- letos fósiles prácticamente completos del Necrolestes. El es- En 1891, Florentino Ameghino (1854-1911) dio a tudio de esos esqueletos llevó a pensar que se trataba de conocer unos restos fósiles encontrados por su hermano un mamífero muy arcaico en la historia de la evolución, Carlos (1865-1936) en las barrancas de Monte Obser- más que un ancestro de los topos, como había supuesto vación, en la provincia de Santa Cruz, en yacimientos Ameghino. Por determinados rasgos se pensó que podía de unos 17 millones de años de antigüedad. Determinó haber sido un marsupial, es decir, un pariente lejano de las que pertenecían a un pequeño –escasos 10cm de largo, comadrejas, los canguros y los coalas actuales. del hocico a la cola– y desconocido mamífero extin- Ciertos investigadores aceptaron esta última hipótesis guido. Estudió los diminutos huesos y consideró que de parentesco, pero otros se mostraron escépticos acerca el animal habría sido un pariente lejano de los topos de ella y se inclinaron por considerar inciertas las rela- africanos vivientes. Le dio el nombre científicoN ecrolestes ciones genealógicas del diminuto mamífero. Así, su po- patagonensis, es decir, ladrón de tumbas de la Patagonia, en sición en el árbol evolutivo de los mamíferos fue objeto alusión a sus hábitos excavadores. El hallazgo, publica- de debate durante gran parte del siglo XX. Para algunos, do por Ameghino en el número de la Revista Argentina de era pariente lejano de las mulitas; otros seguían pensan- Historia Natural citado entre las lecturas sugeridas, atrajo do que podía estar relacionado con los topos, y para un la atención del ámbito científico, ya que hasta ese mo- tercer grupo, sus vínculos eran con los marsupiales aus- mento en Sudamérica no se habían encontrado restos tralianos. -

0 Introduction
Cambridge University Press 978-0-521-78117-6 - Evolution of Tertiary Mammals of North America, Volume 2: Small Mammals, Xenarthrans, and Marine Mammals Christine M. Janis, Gregg F. Gunnell and Mark D. Uhen Excerpt More information 0 Introduction christine m. janis,1 gregg f. gunnell2 and mark d. uhen3 1Brown University, Providence, RI, USA 2Museum of Paleontology, University of Michigan, Ann Arbor, MI, USA 3Smithsonian Institution, Washington, DC, USA AIMS OF VOLUME 2 the chapter are presented according to a standardized format, and the institutional abbreviations have also been standardized and are This enterprise was originally conceived of as a single volume. How- listed in an appendix (Appendix III). ever, after a span of 10 years from its original conception, the current senior editor (Christine Janis), and the then junior editors (Kathleen Scott and Louis Jacobs) realized that it would be more realistic THE STANDARDIZED LAYOUT OF EACH CHAPTER to proceed with chapters then in hand, which could more or less be assembled into the conceptually useful, if taxonomically para- The chapters are laid out in a similar fashion to those in Volume 1. phyletic, rubric of “Terrestrial Carnivores and Ungulates” (Janis, The contributors were requested to adhere to a common layout for Scott, and Jacobs, 1998). This in part reflected the chapters that had each chapter, in order to provide uniform information throughout been assembled to date, although it should be noted that some of the book. The “Introduction” for each chapter introduces the group. the chapters in this current volume, most notably those by Darryl The “Defining features” section lays out the basic cranial, dental, Domning on sirenians and desmostylians, were among the first ones and postcranial features of the taxon. -

The Oldest Platypus and Its Bearing on Divergence Timing of the Platypus and Echidna Clades
The oldest platypus and its bearing on divergence timing of the platypus and echidna clades Timothy Rowe*†, Thomas H. Rich‡§, Patricia Vickers-Rich§, Mark Springer¶, and Michael O. Woodburneʈ *Jackson School of Geosciences, University of Texas, C1100, Austin, TX 78712; ‡Museum Victoria, PO Box 666, Melbourne, Victoria 3001, Australia; §School of Geosciences, PO Box 28E, Monash University, Victoria 3800, Australia; ¶Department of Biology, University of California, Riverside, CA 92521; and ʈDepartment of Geology, Museum of Northern Arizona, Flagstaff, AZ 86001 Edited by David B. Wake, University of California, Berkeley, CA, and approved October 31, 2007 (received for review July 7, 2007) Monotremes have left a poor fossil record, and paleontology has broadly affect our understanding of early mammalian history, been virtually mute during two decades of discussion about with special implications for molecular clock estimates of basal molecular clock estimates of the timing of divergence between the divergence times. platypus and echidna clades. We describe evidence from high- Monotremata today comprises five species that form two resolution x-ray computed tomography indicating that Teinolo- distinct clades (16). The echidna clade includes one short-beaked phos, an Early Cretaceous fossil from Australia’s Flat Rocks locality species (Tachyglossus aculeatus; Australia and surrounding is- (121–112.5 Ma), lies within the crown clade Monotremata, as a lands) and three long-beaked species (Zaglossus bruijni, Z. basal platypus. Strict molecular clock estimates of the divergence bartoni, and Z. attenboroughi, all from New Guinea). The between platypus and echidnas range from 17 to 80 Ma, but platypus clade includes only Ornithorhynchus anatinus (Austra- Teinolophos suggests that the two monotreme clades were al- lia, Tasmania). -

Mammals at Woodland Park Zoo Pre-Visit Information
Mammals at Woodland Park Zoo Pre-visit Information If you are planning a zoo field trip and wish to have your students focus on mammals during their visit, this pre- visit sheet can help them get the most out of their time at the zoo. We have put together an overview of key concepts related to mammals, a list of basic vocabulary words, and a checklist of mammal species at Woodland Park Zoo. Knowledge and understanding of these main ideas will enhance your students’ zoo visit. OVERVIEW: There are over 5,000 species of mammals currently identified worldwide, inhabiting a number of different biomes and exhibiting a range of adaptations. Woodland Park Zoo exhibits a wide variety of mammal species (see attached checklist) in several different areas of the zoo. A mammal field trip to the zoo could focus on the characteristics of mammals (see “Concepts” below), comparing/contrasting different mammals or learning about biomes and observing the physical characteristics of mammals in different biomes. CONCEPTS: Mammals share the following physical characteristics: • Fur or hair • Endothermic, often called warm-blooded. Endothermic animals maintain a constant internal body temperature rather than adjusting to the temperature of their surroundings as ectothermic animals (such as reptiles and amphibians) do. • Mammary glands, which are used to feed milk to young Mammals, like all plants and animals, have five basic needs to survive—food, water, shelter, air and space. They inhabit every continent on the planet and range in size from Kitti’s hog-nosed bat (also called bumblebee bat) at 0.07 ounces (2 grams) to the blue whale at 100 tons (approximately 90,000 kilograms). -

Lower Triassic Postcanine Teeth with Allotherian-Like Crowns
Research Letters South African Journal of Science 103, May/June 2007 245 Lower Triassic postcanine teeth with allotherian-like crowns F. Abdala*‡, H. Mocke*§ and P.J. Hancox* The Allotheria are fossil mammals with upper and lower post- canines usually showing two longitudinal rows of cusps separated by a central valley. The group comprises the poorly known haramiyids, mostly represented by isolated teeth, and the notably diverse and long-lived multituberculates; its monophyly is uncer- tain. The oldest records of this particular group are the Late Triassic (Norian–Rhaetian) haramiyids. We present here postcanines with haramiyid-like crowns that were recovered from the Lower Triassic of South Africa. A distinguishing feature of the new teeth is that they are single-rooted. This is the oldest record of mammal-like teeth with crowns having parallel rows of cusps, representing a temporal extension of some 43 million years from similar crown patterns of haramiyids and tritylodontids. This finding reinforces evidence of the remarkable faunal turnover of therapsids in the Early/Middle Triassic, at which time an explosive origin followed by a rapid early diversification of herbivorous/omnivorous forms with occluding expanded postcanines took place. Introduction The Beaufort Group of the South African Karoo shows an abundance and diversity of non-mammalian synapsids, which have allowed for biostratigraphic subdivisions ranging from Middle Permian to Middle Triassic.1 The youngest of these Fig. 1.Allotherian-like teeth.A, Occlusal and lateral views of BP/1/6515 (Pattern 1); B, occlusal and lateral views of BP/1/6516 (Pattern 2). biozones, the Cynognathus Assemblage Zone (AZ), comprises the full extent of the Burgersdorp Formation of the Tarkastad Sub- found to be most parsimonious from an unconstrained search, group (J. -

Early Cretaceous Amphilestid ('Triconodont') Mammals from Mongolia
Early Cretaceous amphilestid ('triconodont') mammals from Mongolia ZOFIAKIELAN-JAWOROWSKA and DEMBERLYIN DASHZEVEG Kielan-Jaworowską Z. &Daslueveg, D. 1998. Early Cretaceous amphilestid (.tricono- dont') mammals from Mongotia. - Acta Pal.aeontol.ogicaPolonica,43,3, 413438. Asmall collection of ?Aptianor ?Albian amphilestid('triconodont') mammals consisting of incomplete dentaries and maxillae with teeth, from the Khoboor localiĘ Guchin Us counĘ in Mongolia, is described. Grchinodon Troftmov' 1978 is regarded a junior subjective synonym of GobiconodonTroftmov, 1978. Heavier wear of the molariforms M3 andM4than of themore anteriorone-M2 in Gobiconodonborissiaki gives indirect evidence formolariformreplacement in this taxon. The interlocking mechanismbetween lower molariforms n Gobiconodon is of the pattern seen in Kuchneotherium and Ttnodon. The ińterlocking mechanism and the type of occlusion ally Amphilestidae with Kuehneotheriidae, from which they differ in having lower molariforms with main cusps aligned and the dentary-squamosal jaw joint (double jaw joint in Kuehneotheńdae). The main cusps in upper molariforms M3-M5 of Gobiconodon, however, show incipient tńangular arrangement. The paper gives some support to Mills' idea on the therian affinities of the Amphilestidae, although it cannot be excluded that the characters that unite the two groups developed in parallel. Because of scanty material and arnbiguĘ we assign the Amphilestidae to order incertae sedis. Key words : Mammali4 .triconodonts', Amphilestidae, Kuehneotheriidae, Early Cretaceous, Mongolia. Zofia Kiel,an-Jaworowska [zkielnn@twarda,pan.pl], InsĘtut Paleobiologii PAN, ul. Twarda 5 I /5 5, PL-00-8 I 8 Warszawa, Poland. DemberĘin Dash7eveg, Geological Institute, Mongolian Academy of Sciences, Ulan Bator, Mongolia. Introduction Beliajeva et al. (1974) reportedthe discovery of Early Cretaceous mammals at the Khoboor locality (referred to also sometimes as Khovboor), in the Guchin Us Soinon (County), Gobi Desert, Mongolia. -

Evolution of Nervous Systems and Brains 2
Evolution of Nervous Systems and Brains 2 Gerhard Roth and Ursula Dicke The modern theory of biological evolution, as estab- drift”) is incomplete; they point to a number of other lished by Charles Darwin and Alfred Russel Wallace and perhaps equally important mechanisms such as in the middle of the nineteenth century, is based on (i) neutral gene evolution without natural selection, three interrelated facts: (i) phylogeny – the common (ii) mass extinctions wiping out up to 90 % of existing history of organisms on earth stretching back over 3.5 species (such as the Cambrian, Devonian, Permian, and billion years, (ii) evolution in a narrow sense – Cretaceous-Tertiary mass extinctions) and (iii) genetic modi fi cations of organisms during phylogeny and and epigenetic-developmental (“ evo - devo ”) self-canal- underlying mechanisms, and (iii) speciation – the ization of evolutionary processes [ 2 ] . It remains uncer- process by which new species arise during phylogeny. tain as to which of these possible processes principally Regarding the phylogeny, it is now commonly accepted drive the evolution of nervous systems and brains. that all organisms on Earth are derived from a com- mon ancestor or an ancestral gene pool, while contro- versies have remained since the time of Darwin and 2.1 Reconstruction of the Evolution Wallace about the major mechanisms underlying the of Nervous Systems and Brains observed modi fi cations during phylogeny (cf . [1 ] ). The prevalent view of neodarwinism (or better In most cases, the reconstruction of the evolution of “new” or “modern evolutionary synthesis”) is charac- nervous systems and brains cannot be based on fossil- terized by the assumption that evolutionary changes ized material, since their soft tissues decompose, but are caused by a combination of two major processes, has to make use of the distribution of neural traits in (i) heritable variation of individual genomes within a extant species. -

Eutheria (Placental Mammals)
Eutheria (Placental Introductory article Mammals) Article Contents . Introduction J David Archibald, San Diego State University, San Diego, California, USA . Basic Design . Taxonomic and Ecological Diversity Eutheria includes one of three major clades of mammals, the extant members of which are . Fossil History and Distribution referred to as placentals. Phylogeny Introduction have supernumerary teeth (e.g. some whales, armadillos, Eutheria (or Placentalia) is the most taxonomically diverse etc.), in extant placentals the number of teeth is at most of three branches or clades of mammals, the other two three upper and lower incisors, one upper and lower being Metatheria (or Marsupialia) and Prototheria (or canine, four upper and lower premolars, and three upper Monotremata). When named by Gill in 1872, Eutheria and lower molars. Except for one fewer upper molar, a included both marsupials and placentals. It was Huxley in domestic dog retains this pattern. Compared to reptiles, 1880 that recognized Eutheria basically as used today to mammals have fewer skull bones through fusion and loss, include only placentals. McKenna and Bell in their although bones are variously emphasized in each of the Classification of Mammals, published in 1997, chose to three major mammalian taxa. use Placentalia rather than Eutheria to avoid the confusion Physiologically, mammals are all endotherms of varying of what taxa should be included in Eutheria. Others such as degrees of efficiency. They are also homeothermic with a Rougier have used Eutheria and Placentalia in the sense relatively high resting temperature. These characteristics used here. Placentalia includes all extant placentals and are also found in birds, but because of anatomical their most recent common ancestor. -
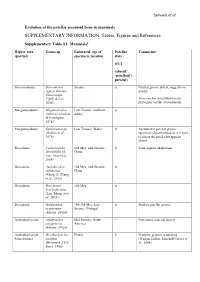
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels et al. Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammals$ Higher taxa Genus sp. Estimated. age of Patellar Comments# (partial) specimen, location state 0/1/2 (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska, Cifelli & Luo, Sinoconodon is included on our 2004) phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji, China Luo, Yuan et al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius China (Meng, Ji, Zhang et al., 2015) Docodonta Docofossor 160 Mya 0 brachydactylus (Luo, Meng, Ji et al., 2015) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Samuels et al. Australosphenida Tachyglossus + Extant 2 Echidnas Monotremata Zaglossus spp. (Herzmark, 1938; Rowe, 1988) Mammaliaformes Fruitafossor 150 Mya, Late Jurassic, 0 Phylogenetic status uncertain indet. windscheffeli (Luo Colorado & Wible, 2005) Mammaliaformes Volaticotherium Late Jurassic/Early ? Hindlimb material incomplete indet. antiquus (Meng, Cretaceous Hu, Wang et al., 2006) Eutriconodonta Jeholodens 120-125 Mya, Early 0 Poorly developed patellar groove jenkinsi (Ji, Luo Cretaceous, China & Ji, 1999) Eutriconodonta Gobiconodon spp. -

Two New Species of Gobiconodon (Mammalia, Eutriconodonta, Gobiconodontidae) from the Lower Cretaceous Shahai and Fuxin Formations, Northeastern China
Historical Biology An International Journal of Paleobiology ISSN: 0891-2963 (Print) 1029-2381 (Online) Journal homepage: http://www.tandfonline.com/loi/ghbi20 Two new species of Gobiconodon (Mammalia, Eutriconodonta, Gobiconodontidae) from the Lower Cretaceous Shahai and Fuxin formations, northeastern China Nao Kusuhashi, Yuan-Qing Wang, Chuan-Kui Li & Xun Jin To cite this article: Nao Kusuhashi, Yuan-Qing Wang, Chuan-Kui Li & Xun Jin (2016) Two new species of Gobiconodon (Mammalia, Eutriconodonta, Gobiconodontidae) from the Lower Cretaceous Shahai and Fuxin formations, northeastern China, Historical Biology, 28:1-2, 14-26 To link to this article: http://dx.doi.org/10.1080/08912963.2014.977881 Published online: 01 Oct 2015. Submit your article to this journal View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=ghbi20 Download by: [University of Sussex Library] Date: 01 October 2015, At: 18:24 Historical Biology, 2016 Vol. 28, Nos. 1–2, 14–26, http://dx.doi.org/10.1080/08912963.2014.977881 Two new species of Gobiconodon (Mammalia, Eutriconodonta, Gobiconodontidae) from the Lower Cretaceous Shahai and Fuxin formations, northeastern China Nao Kusuhashia*, Yuan-Qing Wangb, Chuan-Kui Lib and Xun Jinb aDepartment of Earth’s Evolution and Environment, Graduate School of Science and Engineering, Ehime University, Ehime 790-8577, Japan; bKey Laboratory of Vertebrate Evolution and Human Origins of Chinese Academy of Sciences, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing 100044, P.R. China (Received 29 July 2014; accepted 14 October 2014) Two new gobiconodontid mammals, Gobiconodon tomidai sp.