3: Biopolymer Research and Development in Europe and Japan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Whither the Keiretsu, Japan's Business Networks? How Were They Structured? What Did They Do? Why Are They Gone?
IRLE IRLE WORKING PAPER #188-09 September 2009 Whither the Keiretsu, Japan's Business Networks? How Were They Structured? What Did They Do? Why Are They Gone? James R. Lincoln, Masahiro Shimotani Cite as: James R. Lincoln, Masahiro Shimotani. (2009). “Whither the Keiretsu, Japan's Business Networks? How Were They Structured? What Did They Do? Why Are They Gone?” IRLE Working Paper No. 188-09. http://irle.berkeley.edu/workingpapers/188-09.pdf irle.berkeley.edu/workingpapers Institute for Research on Labor and Employment Institute for Research on Labor and Employment Working Paper Series (University of California, Berkeley) Year Paper iirwps-- Whither the Keiretsu, Japan’s Business Networks? How Were They Structured? What Did They Do? Why Are They Gone? James R. Lincoln Masahiro Shimotani University of California, Berkeley Fukui Prefectural University This paper is posted at the eScholarship Repository, University of California. http://repositories.cdlib.org/iir/iirwps/iirwps-188-09 Copyright c 2009 by the authors. WHITHER THE KEIRETSU, JAPAN’S BUSINESS NETWORKS? How were they structured? What did they do? Why are they gone? James R. Lincoln Walter A. Haas School of Business University of California, Berkeley Berkeley, CA 94720 USA ([email protected]) Masahiro Shimotani Faculty of Economics Fukui Prefectural University Fukui City, Japan ([email protected]) 1 INTRODUCTION The title of this volume and the papers that fill it concern business “groups,” a term suggesting an identifiable collection of actors (here, firms) within a clear-cut boundary. The Japanese keiretsu have been described in similar terms, yet compared to business groups in other countries the postwar keiretsu warrant the “group” label least. -

D0ra02344b1.Pdf
Electronic Supplementary Material (ESI) for RSC Advances. This journal is © The Royal Society of Chemistry 2020 Supporting Information Multifaceted Property Tailoring of Polyamide 6 by Blending Miscible and Immiscible components: Ternary Blends of Polyamide 6/Polyethylene Terephthalate/Phenol Novolac Takayuki Hiraia,*, Yusaku Onochib, Jumpei Kawadaa aMaterial and Processing Department, Polymer Processing and Mechanics Laboratories, Toyota Central R&D Laboratories, Inc., 41-1 Yokomichi, Nagakute 480-1192, Japan bLightweight Material Development Group, Organic Material Dept., Organic Material Engineering Div., Toyota Motor Corporation, Toyota-cho, Toyota, Aichi, Japan *Corresponding Author. Email: [email protected] Abbreviations PA6: Polyamide 6, PBT: poly(butylene terephthalate), PEN: poly(ethylene naphthalate), PEI: poly(ether imide), PN: phenol novolac 1 Figure S1. Temperature dependencies of (a) the storage modulus and (b) tan δ of PA6 in dry state after vacuum drying and water-absorbed state after reaching equivalent state in water. The water molecules act as a plasticizer and the glass transition temperature (Tg) of PA6 is low- temperature shifted by water absorption. Figure S2. (a) Temperature dependencies of the storage modulus and (b) flexural moduli of PA/PET and ternary blends in water-absorbed state (PA6/PET/PN5:PA6/PET including 5 wt% PN and PA6/PET/PN15: including 15 wt% PN.) 2 Figure S3. (a) Viscoelastic behavior and (b) stress–strain curves of PA6 and the polymer blends, which were obtained from vacuum-dried specimens. Figure S4. Viscoelastic behavior of homo PA6, homo PBT, and the polymer blends in water- absorbed state. Temperature dependencies of (a) the storage modulus and (b) tan δ. 3 Figure S5. -
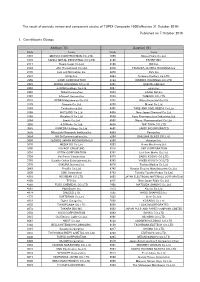
Published on 7 October 2016 1. Constituents Change the Result Of
The result of periodic review and component stocks of TOPIX Composite 1500(effective 31 October 2016) Published on 7 October 2016 1. Constituents Change Addition( 70 ) Deletion( 60 ) Code Issue Code Issue 1810 MATSUI CONSTRUCTION CO.,LTD. 1868 Mitsui Home Co.,Ltd. 1972 SANKO METAL INDUSTRIAL CO.,LTD. 2196 ESCRIT INC. 2117 Nissin Sugar Co.,Ltd. 2198 IKK Inc. 2124 JAC Recruitment Co.,Ltd. 2418 TSUKADA GLOBAL HOLDINGS Inc. 2170 Link and Motivation Inc. 3079 DVx Inc. 2337 Ichigo Inc. 3093 Treasure Factory Co.,LTD. 2359 CORE CORPORATION 3194 KIRINDO HOLDINGS CO.,LTD. 2429 WORLD HOLDINGS CO.,LTD. 3205 DAIDOH LIMITED 2462 J-COM Holdings Co.,Ltd. 3667 enish,inc. 2485 TEAR Corporation 3834 ASAHI Net,Inc. 2492 Infomart Corporation 3946 TOMOKU CO.,LTD. 2915 KENKO Mayonnaise Co.,Ltd. 4221 Okura Industrial Co.,Ltd. 3179 Syuppin Co.,Ltd. 4238 Miraial Co.,Ltd. 3193 Torikizoku co.,ltd. 4331 TAKE AND GIVE. NEEDS Co.,Ltd. 3196 HOTLAND Co.,Ltd. 4406 New Japan Chemical Co.,Ltd. 3199 Watahan & Co.,Ltd. 4538 Fuso Pharmaceutical Industries,Ltd. 3244 Samty Co.,Ltd. 4550 Nissui Pharmaceutical Co.,Ltd. 3250 A.D.Works Co.,Ltd. 4636 T&K TOKA CO.,LTD. 3543 KOMEDA Holdings Co.,Ltd. 4651 SANIX INCORPORATED 3636 Mitsubishi Research Institute,Inc. 4809 Paraca Inc. 3654 HITO-Communications,Inc. 5204 ISHIZUKA GLASS CO.,LTD. 3666 TECNOS JAPAN INCORPORATED 5998 Advanex Inc. 3678 MEDIA DO Co.,Ltd. 6203 Howa Machinery,Ltd. 3688 VOYAGE GROUP,INC. 6319 SNT CORPORATION 3694 OPTiM CORPORATION 6362 Ishii Iron Works Co.,Ltd. 3724 VeriServe Corporation 6373 DAIDO KOGYO CO.,LTD. 3765 GungHo Online Entertainment,Inc. -

1 Atb 2016-III Consolidated Business of Fiber & Textile Makers in Japan, April 1, 2019 to March 31, 2020
Consolidated Business of Fiber & Textile Makers in Japan, April 1, 2019 to March 31, 2020 (million yen) Y-o-Y Operating Y-o-Y Y-o-Y Net Sales Net Profits Change (%) Profits Change (%) Change (%) Fiber producers Toray 2,214,633 –7.3 131,186 –7.3 55,725 –29.8 Asahi Kasei 2,151,646 –0.9 177,264 –15.4 103,931 –29.5 Teijin 853,746 –3.9 56,205 –6.3 25,252 –44.0 Kaneka 601,514 –3.1 26,014 –27.8 14,003 –37.0 Toyobo 339,607 0.9 22,794 4.9 13,774 — Unitika 119,537 –7.4 5,467 –32.9 –2,158 — Kuraray(1) 575,807 –4.5 54,173 –17.7 –1,956 — Cotton spinners & textile makers Daiwabo Holdings 944,053 20.2 32,841 44.6 21,178 26.2 Kurabo 142,926 –9.0 4,541 –19.5 3,731 –19.7 Shikibo 38,037 –6.8 1,979 –17.7 983 — Fujibo Holdings 38,701 4.3 4,079 7.9 2,269 –10.6 Omikenshi 9,026 –7.4 –207 — –2,367 — Nitto Boseki 85,722 4.2 8,160 –0.5 5,771 –27.7 Nisshinbo Holdings(2) 509,660 — 6,284 — –6,604 — Textile dyers & processors Seiren 120,258 –2.0 10,502 –0.8 8,551 3.9 Komatsu Matere 36,525 –6.5 1,612 –25.5 1,375 –35.5 Sakai Ovex 27,561 1.1 2,123 4.9 2,313 3.8 Tokai Senko 14,010 –3.4 617 –17.9 –551 — Sotoh 11,219 –0.1 193 –19.1 –97 — Soko Seiren 2,778 –17.7 –245 — –130 — Note: (1) Kuraray's business performance is for the period from January to December 2019. -

Whither the Keiretsu, Japan's Business Networks? How Were They Structured? What Did They Do? Why Are They Gone?
UC Berkeley Working Paper Series Title Whither the Keiretsu, Japan's Business Networks? How Were They Structured? What Did They Do? Why Are They Gone? Permalink https://escholarship.org/uc/item/00m7d34g Authors Lincoln, James R. Shimotani, Masahiro Publication Date 2009-09-24 eScholarship.org Powered by the California Digital Library University of California WHITHER THE KEIRETSU, JAPAN’S BUSINESS NETWORKS? How were they structured? What did they do? Why are they gone? James R. Lincoln Walter A. Haas School of Business University of California, Berkeley Berkeley, CA 94720 USA ([email protected]) Masahiro Shimotani Faculty of Economics Fukui Prefectural University Fukui City, Japan ([email protected]) 1 INTRODUCTION The title of this volume and the papers that fill it concern business “groups,” a term suggesting an identifiable collection of actors (here, firms) within a clear-cut boundary. The Japanese keiretsu have been described in similar terms, yet compared to business groups in other countries the postwar keiretsu warrant the “group” label least. The prewar progenitor of the keiretsu, the zaibatsu, however, could fairly be described as groups, and, in their relatively sharp boundaries, hierarchical structure, family control, and close ties to the state were structurally similar to business groups elsewhere in the world. With the break-up by the U. S. Occupation of the largest member firms, the purging of their executives, and the outlawing of the holding company structure that held them together, the zaibatsu were transformed into quite different business entities, what we and other literature call “network forms” of organization (Podolny and Page, 1998; Miyajima, 1994). -

PLA As a Packaging Solution Introduction to PLA – Why It Is Being Considered a Potential Material?
PLA as a Packaging Solution Introduction to PLA – Why it is being considered a potential material? What is PLA? Polylactic acid, or polylactide (PLA) is a thermoplastic polyester formally obtained by condensation of lactic acid with loss of water (hence its name). PLA has become a popular material due to it being economically produced from renewable resources. In 2010, PLA had the second highest consumption volume of any bioplastic of the world. Widely used for filament material in 3D printing, it is now considered in packaging applications, especially in food packaging. And, it is anticipated that the growing consumption of packaged foods will further drive the packaging industry to use this as a primary biodegradable material over the coming years - global market for PLA is expected to become more than US$ 5 billion by 2020 [Source]. BIODEGRADABLE GOOD BARRIER RENEWABLE LOW COST PLA is biodegradable under PROPERTIES Being derived from plants, PLA is certain circumstances [source], as Good moisture barrier properties completely bio based i.e. the enzymes like Proteinase K, which can comparable to those of petroleum- original materials are renewable. catalyze the hydrolytic degradation based plastics, such as polyethylene [Source] of PLA, are not available in the terephthalate (PET) or polystyrene environment except on rare occasions. That is why there has (PS) [Source] been research on various blends of PLA to improve its biodegradability [please refer to the subsequent slides for more information on this]. GreyB Comment: The high market and research in PLA is a direct signal of it growing even bigger in future – thus, good chances are that it will also be popular as a packaging alternative. -

METAL JAPAN You Can Enter All Concurrent Shows with This Ticket
This is a SAMPLE. Please request actual exhibition tickets from here. ▶▶▶ http://www.metal-japan.jp/en/inv/pre/ INVITATION TICKET Japan’s Largest*1 ! 170 Exhibitors Held inside Highly-functional Material Week 2017 50 Exhibitors Newly Exhibiting! 170 Exhibitors Gather 4th The numbers of exhibitors (including co-exhibitors), visitors and countries on this invitation ticket are forecast released on December 2, 2016. These numbers may differ from actual numbers at the show. *1 In highly-functional metal industry. *2 Including concurrent shows. *3 Including regions and concurrent shows. Covering All Advanced Metals & Technologies! Concurrent Shows 1,540 Exhibitors in total METAL JAPAN You can enter all concurrent shows with this ticket. East Hall 4–8 Floor Plan (Preliminary) METAL JAPAN Exhibitors ー Highly-functional Metal Expo ー 170 Highly-functional Material Week 850 Exhibitors Dates: April 5 [Wed] – 7 [Fri ] , 2017 10:00–18:00 (10:00–17:00 on Apr. 7) Cordially invited by: Organiser NEW Venue: Tokyo Big Sight, Japan Reed Exhibitions Japan Ltd. 2nd 4th 1st 6th 8th Office address: Organised by: Reed Exhibitions Japan Ltd. 18F Shinjuku-Nomura Bldg., Inspection/Analysis Processing Equipment Processing Technology CERAMICS METAL JOINING PLASTIC FilmTech 1-26-2 Nishishinjuku, Shinjuku-ku, Web: www.metal-japan.jp/en/ Tokyo 163-0570, Japan JAPAN JAPAN JAPAN JAPAN JAPAN • Non-destructive Inspection • Pressing Machine • Cutting Machine • Casting/Forging • Sheet Metal Working This ticket admits one person only. This exhibition is primarily open to trade. All visitors are required to bring an invitation ticket and • Material Analysis • Machining Center • Die-casting Machine • Die-casting • Powder Metallurgy 140 Exhibitors 170 Exhibitors 110 Exhibitors 180 Exhibitors 250 Exhibitors 2 business cards. -
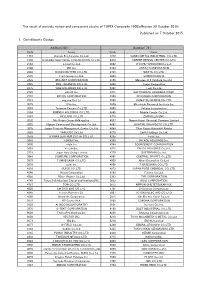
Published on 7 October 2015 1. Constituents Change the Result Of
The result of periodic review and component stocks of TOPIX Composite 1500(effective 30 October 2015) Published on 7 October 2015 1. Constituents Change Addition( 80 ) Deletion( 72 ) Code Issue Code Issue 1712 Daiseki Eco.Solution Co.,Ltd. 1972 SANKO METAL INDUSTRIAL CO.,LTD. 1930 HOKURIKU ELECTRICAL CONSTRUCTION CO.,LTD. 2410 CAREER DESIGN CENTER CO.,LTD. 2183 Linical Co.,Ltd. 2692 ITOCHU-SHOKUHIN Co.,Ltd. 2198 IKK Inc. 2733 ARATA CORPORATION 2266 ROKKO BUTTER CO.,LTD. 2735 WATTS CO.,LTD. 2372 I'rom Group Co.,Ltd. 3004 SHINYEI KAISHA 2428 WELLNET CORPORATION 3159 Maruzen CHI Holdings Co.,Ltd. 2445 SRG TAKAMIYA CO.,LTD. 3204 Toabo Corporation 2475 WDB HOLDINGS CO.,LTD. 3361 Toell Co.,Ltd. 2729 JALUX Inc. 3371 SOFTCREATE HOLDINGS CORP. 2767 FIELDS CORPORATION 3396 FELISSIMO CORPORATION 2931 euglena Co.,Ltd. 3580 KOMATSU SEIREN CO.,LTD. 3079 DVx Inc. 3636 Mitsubishi Research Institute,Inc. 3093 Treasure Factory Co.,LTD. 3639 Voltage Incorporation 3194 KIRINDO HOLDINGS CO.,LTD. 3669 Mobile Create Co.,Ltd. 3197 SKYLARK CO.,LTD 3770 ZAPPALLAS,INC. 3232 Mie Kotsu Group Holdings,Inc. 4007 Nippon Kasei Chemical Company Limited 3252 Nippon Commercial Development Co.,Ltd. 4097 KOATSU GAS KOGYO CO.,LTD. 3276 Japan Property Management Center Co.,Ltd. 4098 Titan Kogyo Kabushiki Kaisha 3385 YAKUODO.Co.,Ltd. 4275 Carlit Holdings Co.,Ltd. 3553 KYOWA LEATHER CLOTH CO.,LTD. 4295 Faith, Inc. 3649 FINDEX Inc. 4326 INTAGE HOLDINGS Inc. 3660 istyle Inc. 4344 SOURCENEXT CORPORATION 3681 V-cube,Inc. 4671 FALCO HOLDINGS Co.,Ltd. 3751 Japan Asia Group Limited 4779 SOFTBRAIN Co.,Ltd. 3844 COMTURE CORPORATION 4801 CENTRAL SPORTS Co.,LTD. -

Diversified Expansion and Different Business Models in the Japanese Textile Industry
Volume 3, Issue 4, Winter 2004 DIVERSIFIED EXPANSION AND DIFFERENT BUSINESS MODELS IN THE JAPANESE TEXTILE INDUSTRY ASLI M. COLPAN Kyoto Institute of Technology, Graduate School of Advanced Fibro-science and Kyoto University, Graduate School of Economics ABSTRACT Diversification into non-textile activities became the major instrument for Japan’s large textile enterprises which confronted the maturing state of their original businesses. Diversification strategies that companies adopted exhibited the different directions in their basic investment patterns. The present research confirms that dissimilar technological resource and capability endownments have decisive impacts on the contrasting long-term growth patterns of the companies. The timing of new market entry is also endogenous to the firm resources and capabilities. While firms adapt to the changing environments, diversification can thus be a very much path-dependent process. Keywords: textile industry, Japan, technological resources and capabilities, diversification strategy 1. Introduction on the basic directions of the diversification strategies in the Japanese textile industry. Diversification into non-textile product It thus explores the diversification patterns markets has been the primary strategy for of the leading companies’ and in particular reorganization adapted by the large analyzes the effects of their dissimilar enterprises in the Japanese textile industry.1 technological resources and capabilities on This was because restructuring within textile the different paths of diversification. businesses has not yielded the long-term solution to bring the satisfactory financial For the purposes of this analysis, the outcomes. As a result, the average textile paper uses internal resource-base theories of sales of the largest firms in the Japanese the firm (Penrose, 1959; Nelson and Winter, textile industry have declined from an 1982), which suggests that firms shall average of ninety percent in 1970 to around exploit their resources and capabilities to only forty percent in the present day. -

Oct 6 -10 , 2020
Industrial Plastic & Rubber Components Procurement Raw Materials, Expo for Automobile, Additives & Fillers Expo Electronics & Medical Contracted Manufacturing & Plastic Molding Processing Service Expo Machines & System Expo Recycling Equipment Expo Mold Design & Manufacturing System Expo 111.042 mm Foamed Plastic Expo Rubber Materials & Co-organizer. Foam Times Co., Ltd. Molding System Expo Composite Materials & Molding System Expo -FRP & CFRP- Once in 3 years! Japan’s biggest Oct 6 [ TUE ] -10 [ SAT ] , 2020 10:00-17:00 [ Last day, closed 16:00] plastic & rubber exhibition Venue: Makuhari Messe, Hall 1-8 The exhibition attracts more than 50,000 professionals in the plastics & rubber industry. IPF Japan www.ipapan.jp (Number of people) IPF 2017 Japanese Overseas Total Space Reservation Information Visitors 40,110 3,566 43,676 Exhibitors 9,131 950 10,081 Total 49,241 4,516 53,757 About IPF Japan An Exhibition Specializing in Plastics and Rubber. More than 50,000 plastics & rubber IPF Japan 2017 (Number of people) industry professionals visit from Japan Japanese Overseas Total and overseas during the 5-day Visitors 40,110 3,566 43,676 * exhibition period. Exhibitors 9,131 950 10,081 Total 49,241 4,516 53,757 *The figures are the number of exhibitor’s entry badges issued at the registration. Japan’s Largest Manufacturing Exhibition. The Japan’s leading manufacturing exhibit IPF Japan 2017 with 778 exhibitors at Makuhari Messe Total Area 54,000㎡ International Exhibition Halls 1-8. Exhibitors 778 companies Booth Units 2,438 units It Covers Every Process of Plastic and Rubber Molding. Exhibitors’ fields cover every process in plastics and rubber, including raw material, processing machinery, manufacturing and recycling. -

UNITIKA CSR Report 2009 UNITIKA Group Corporate Social Responsibility Report 2009 CONTENTS
UNITIKA CSR Report 2009 UNITIKA Group Corporate Social Responsibility Report 2009 CONTENTS Management Message from the President 2 Notes on FY 2009 Report 3 Company Overview 4 Management Philosophy 5 Corporate Governance 5 Internal Control 6 CSR Promotion System 7 Environment, Health, and Safety (EHS) Management 7 Compliance Promotion 8 Information Security Management 8 CSR Report Relationships with Clients 9 Ensuring Product Safety 9 Quality Assurance Activities 9 Relationships with Shareholders & Investors 10 IR Activities 10 Share Status 10 Giving Back to the Community 11 Efforts Toward Environmental Improvement 11 Disaster Prevention & Readiness Efforts 12 Public Relations Activities 13 Report on Promotional Event Held at the Unitika Fitness Club in Uji, Kyoto 14 Concern for Our Employees 16 Personnel System 16 Equal Opportunity 16 Human Resource Development 17 Employee Mental Health 17 Human Rights 17 Safety & Health Activities 18 Asbestos Removal 18 Environmental Basic Environmental Policy 19 Report Medium-Term Environmental Plan 20 History of Environmental Preservation Activities 21 Overview of Environmental Impact 22 Work on Reducing Environmental Impact 23 Air Pollution 23 Water Pollution 23 Waste Products 24 Rate of Recycling 24 Handling of Chemical Substances 25 Energy Saving & Global Warming 26 Logistics 27 Environmental Complaints 27 Environmental Accounting 28 Technology and Products for Environmental Safety 29 Water Treatment Facilities 29 Garbage Processing Facilities 30 Air Pollution 30 Plant-Derived Biomass Material / Terramac 31 Anticorrosive Sheeting / Segurova 32 Recycled Polyester Fiber / Uniecolo 33 Plant-Derived Biomass Material / Castlon 33 New Natural Fibers / Sylph 34 Organic Cotton Material / Naturecot 34 Recycled Polyester Nonwoven Sheeting / Ecomix 35 Glass Beads / Unibeads for use in Road Marking 35 Production Site Information 36 CONTENTS 1 Message from the President As a good corporate citizen, we contribute to a healthier environment and a more comfortable way of life. -

Nonwoven Fabric Manufacturers
Nonwoven Fabric Manufacturers Country of Company Product Markets, Customers Contact production Through-out Ahlstrom Munksjö Nonwoven fabrics for scrubs, surgical gowns and masks. Global operations https://www.ahlstrom-munksjo.com/ Europe, USA, Brazil, Other medical products India, South Korea, China USA Atex Nonwoven fabrics for medical textile products. Other https://www.atextechnologies.com/ textile products Germany, Ireland, Freudenberg Nonwoven fabrics and other devices for medical use. Global operations https://www.freudenbergmedical.de/en/ China, USA, Costa Rica, etc. USA + global Berry Global Inc. Nonwoven fabrics for medical garments and other use. https://www.berryglobal.com/ USA + global DuPont Nonwovens like Tyvek for workwear, medical devices, Global https://www.dupont.com/transportation- Nonwovens?? industrial/healthcare.html China Zouping Huaiang Nonwoven Co. SMS and other nonwoven fabrics for medical textiles. http://www.hqnonwoven.com/ Ltd UK Don & Low Nonwovens for medical products, wound care and other https://www.donlow.co.uk/ medical products. Sweden, Denmark, Fibertex Nonwovens Nonwoven fabrics for medical gowns and masks. http://www.fibertex.com Turkey, Czech Republic, France, USA, South Africa Turkey General Nonwovens and Nonwoven fabrics for hygiene products. Medical http://www.generalnonwovens.com Composites nonwovens? Greece Thrace Group Nonwoven fabrics for medical textiles http://www.thraceplastics.gr Japan Asahi Kasei Nonwoven fabrics for medical textiles https://www.asahi-kasei.co.jp/asahi/en/ Brazil, USA, Asia Fitesa Nonwoven fabrics for medical textiles https://www.fitesa.com/en/ Germany Innovatec Specialty nonwovens for medical applications https://melt-blown.com/en/ Switzerland Jakob Holm Specialty nonwovens for medical applications https://www.jacob-holm.com/emea/en-us/ Germany Sandler Nonwovens for medical and other use.