Phytotoxic Metabolites Produced by Legume-Associated Ascochyta and Its Related Genera in the Dothideomycetes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phaeoseptaceae, Pleosporales) from China
Mycosphere 10(1): 757–775 (2019) www.mycosphere.org ISSN 2077 7019 Article Doi 10.5943/mycosphere/10/1/17 Morphological and phylogenetic studies of Pleopunctum gen. nov. (Phaeoseptaceae, Pleosporales) from China Liu NG1,2,3,4,5, Hyde KD4,5, Bhat DJ6, Jumpathong J3 and Liu JK1*,2 1 School of Life Science and Technology, University of Electronic Science and Technology of China, Chengdu 611731, P.R. China 2 Guizhou Key Laboratory of Agricultural Biotechnology, Guizhou Academy of Agricultural Sciences, Guiyang 550006, P.R. China 3 Faculty of Agriculture, Natural Resources and Environment, Naresuan University, Phitsanulok 65000, Thailand 4 Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai 57100, Thailand 5 Mushroom Research Foundation, Chiang Rai 57100, Thailand 6 No. 128/1-J, Azad Housing Society, Curca, P.O., Goa Velha 403108, India Liu NG, Hyde KD, Bhat DJ, Jumpathong J, Liu JK 2019 – Morphological and phylogenetic studies of Pleopunctum gen. nov. (Phaeoseptaceae, Pleosporales) from China. Mycosphere 10(1), 757–775, Doi 10.5943/mycosphere/10/1/17 Abstract A new hyphomycete genus, Pleopunctum, is introduced to accommodate two new species, P. ellipsoideum sp. nov. (type species) and P. pseudoellipsoideum sp. nov., collected from decaying wood in Guizhou Province, China. The genus is characterized by macronematous, mononematous conidiophores, monoblastic conidiogenous cells and muriform, oval to ellipsoidal conidia often with a hyaline, elliptical to globose basal cell. Phylogenetic analyses of combined LSU, SSU, ITS and TEF1α sequence data of 55 taxa were carried out to infer their phylogenetic relationships. The new taxa formed a well-supported subclade in the family Phaeoseptaceae and basal to Lignosphaeria and Thyridaria macrostomoides. -

Nature and Effect of Alternaria Spp. Complex from Wheat Grain on Germination and Disease Transmission
Pak. J. Bot., 45(5): 1817-1824, 2013. NATURE AND EFFECT OF ALTERNARIA SPP. COMPLEX FROM WHEAT GRAIN ON GERMINATION AND DISEASE TRANSMISSION ANALÍA E. PERELLÓ1,2* AND SILVINA LARRÁN1 1CIDEFI (Centro de Investigaciones de Fitopatología) y Cátedra de Fitopatología 2CONICET-Facultad de Ciencias Agrarias y Forestales de la Universidad Nacional de La Plata, Calle 60 y 119 (1900) La Plata, Buenos Aires, Argentina. *Corresponding author’s e-mail: anaperello2@ yahoo.com.ar Abstract Diseases caused by Alternaria sp. are among the most common diseases of crops throughout the world. Alternaria sp. is a common component of the flora of wheat seed. Although isolation of Alternaria sp. from wheat (Triticum aestivum) seed has been reported in Argentina, development of the Alternaria blight in plants from infected seeds has not been demonstrated experimentally. Seed transmission of strains belonging to Alternaria tenuissima, A. alternata, A. infectoria, A. triticina, A. chlamydospora and related genera like Embellisia and Ulocladium sp. on wheat were investigated in the Argentinean growing area, on wheat cultivars Klein Escorpión and Buck Poncho. A. tenuissima was the dominant fungus in black pointed kernels. Transmission of all 42 seed-borne members of Alternaria complex from seeds to seedlings artificially inoculated was detected by trays seedling symptoms test. Among the fungi tested most isolates of Alternaria, Embellisia sp. and Ulocladium sp. produced distinct seed rot and seedling infection symptoms. This confirmed the seed-borne nature of these fungi. In each wheat cultivar tested inoculated seeds appreciably reduced their germination. The emerging coleoptile is externally infected by hyphal growth from the infected pericarp. -

Download Full Article in PDF Format
Cryptogamie, Mycologie, 2013, 34 (4): 303-319 © 2013 Adac. Tous droits réservés Phylogeny and morphology of Leptosphaerulina saccharicola sp. nov. and Pleosphaerulina oryzae and relationships with Pithomyces Rungtiwa PHOOKAMSAK a, b, c, Jian-Kui LIU a, b, Ekachai CHUKEATIROTE a, b, Eric H. C. McKENZIE d & Kevin D. HYDE a, b, c * a Institute of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai 57100, Thailand b School of Science, Mae Fah Luang University, Chiang Rai 57100, Thailand c International Fungal Research & Development Centre, Research Institute of Resource Insects, Chinese Academy of Forestry, Kunming, Yunnan, 650224, China d Landcare Research, Private Bag 92170, Auckland, New Zealand Abstract – A Dothideomycete species, associated with leaf spots of sugarcane (Saccharum officinarum), was collected from Nakhonratchasima Province, Thailand. A single ascospore isolate was obtained and formed the asexual morph in culture. ITS, LSU, RPB2 and TEF1α gene regions were sequenced and analyzed with molecular data from related taxa. In a phylogenetic analysis the new isolate clustered with Leptosphaerulina americana, L. arachidicola, L. australis and L. trifolii (Didymellaceae) and the morphology was also comparable with Leptosphaerulina species. Leptosphaerulina saccharicola is introduced to accommodate this new collection which is morphologically and phylogenetically distinct from other species of Leptosphaerulina. A detailed description and illustration is provided for the new species, which is compared with similar taxa. The type specimen of Pleosphaerulina oryzae, is transferred to Leptosphaerulina. It is redescribed and is a distinct species from L. australis, with which it was formerly synonymized. Leptosphaerulina species have been linked to Pithomyces but the lack of phylogenetic support for this link is discussed. -

Isolation and Identification of Fungi from Leaves Infected with False Mildew on Safflower Crops in the Yaqui Valley, Mexico
Isolation and identification of fungi from leaves infected with false mildew on safflower crops in the Yaqui Valley, Mexico Eber Addi Quintana-Obregón 1, Maribel Plascencia-Jatomea 1, Armando Burgos-Hérnandez 1, Pedro Figueroa-Lopez 2, Mario Onofre Cortez-Rocha 1 1 Departamento de Investigación y Posgrado en Alimentos, Universidad de Sonora, Blvd. Luis Encinas y Rosales s/n, Colonia Centro. C.P. 83000 Hermosillo, Sonora, México. 2 Campo Experimental Norman E. Borlaug-INIFAP. C. Norman Borlaug Km.12 Cd. Obregón, Sonora C.P. 85000 3 1 0 2 Aislamiento e identificación de hongos de las hojas infectadas con la falsa cenicilla , 7 en cultivos de cártamo en el Valle del Yaqui, México 2 - 9 1 Resumen. La falsa cenicilla es una enfermedad que afecta seriamente los cultivos de cártamo en : 7 3 el Valle del Yaqui, México, y es causada por la infección de un hongo perteneciente al género A Ramularia. En el presente estudio, un hongo aislado de hojas contaminadas fue cultivado bajo Í G diferentes condiciones de crecimiento con la finalidad de estudiar su desarrollo micelial y O L producción de esporas, determinándose que el medio sólido de , 18 C de O Septoria tritici ° C I incubación y fotoperiodos de 12 h luz-oscuridad, fueron las condiciones más adecuadas para el M desarrollo del hongo. Este aislamiento fue identificado morfológicamente como Ramularia E D , pero genómicamente como , por lo que no se puede cercosporelloides Cercosporella acroptili A aún concluir que especie causa esta enfermedad. Adicionalmente, en la periferia de las N A C infecciones estudiadas se detectó la presencia de Alternaria tenuissima y Cladosporium I X cladosporioides. -

In Vitro Evaluation of Plant Extracts and Bio- Agents Against Alternaria
International Journal of Chemical Studies 2018; 6(2): 504-507 P-ISSN: 2349–8528 E-ISSN: 2321–4902 IJCS 2018; 6(2): 504-507 In vitro evaluation of plant extracts and bio- © 2018 IJCS Received: 04-01-2018 agents against Alternaria tenuissima (Fr.) keissl Accepted: 05-02-2018 causing leaf blight of kodo millet K Hariprasad Department of Plant Pathology, UAS, GKVK, Bengaluru - 65 K Hariprasad, A Nagaraja, Suresh Patil and Rakesha *Project Coordinating Unit (Small Millets), ICAR, GKVK, Abstract Bengaluru, Karnataka, India Kodo millet (Paspalum scrobiculatum L.) is nutritionally important millet. Leaf blight has been a major production constraint and fungicidal sprays for the management of any disease on this crop may not be A Nagaraja economically viable and feasible as the farmers cultivating the crop are resource poor and the crop is less Department of Plant Pathology, remunerative, but is important in tribal and rainfed agriculture. Hence, botanicals and bio-agents were UAS, GKVK, Bengaluru - 65 *Project Coordinating Unit evaluated in vitro against Alternaria tenuissima the cause of leaf blight. Ten plant extracts and 14 bio- (Small Millets), ICAR, GKVK, agents were evaluated following dual culture. The results revealed 100 per cent inhibition of the mycelial Bengaluru, Karnataka, India growth of A. tenuissima by Eucalyptus sp. and Clerodendron infortunatum at 7.5 and 10.0 per cent concentrations. Among the fungal bio control agents tested, maximum inhibition (100 %) was recorded Suresh Patil in Trichoderma harzianum (NBAIR), followed by T. viride (81.38 %); whereas the bacterial bio agent Department of Plant Pathology, Bacillus amyloliquefaciens (P-42) showed only 77.40 % inhibition of mycelial growth revealing that UAS, GKVK, Bengaluru - 65 fungal antagonists were more effective than the bacterial antagonists. -

Biotechnology of Neglected and Underutilized Crops Biotechnology of Neglected and Underutilized Crops Shri Mohan Jain · S
Shri Mohan Jain · S. Dutta Gupta Editors Biotechnology of Neglected and Underutilized Crops Biotechnology of Neglected and Underutilized Crops Shri Mohan Jain · S. Dutta Gupta Editors Biotechnology of Neglected and Underutilized Crops 1 3 Editors Shri Mohan Jain S. Dutta Gupta Department of Agricultural Sciences Department of Agricultural University of Helsinki and Food Engineering Helsinki Indian Institute of Technology Kharagpur Finland Kharagpur India ISBN 978-94-007-5499-7 ISBN 978-94-007-5500-0 (eBook) DOI 10.1007/978-94-007-5500-0 Springer Dordrecht Heidelberg New York London Library of Congress Control Number: 2013934379 © Springer Science+Business Media Dordrecht 2013 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. Exempted from this legal reservation are brief excerpts in connection with reviews or scholarly analysis or material supplied specifically for the purpose of being entered and executed on a computer system, for exclusive use by the purchaser of the work. Duplication of this publication or parts thereof is permitted only under the provisions of the Copyright Law of the Publisher’s location, in its current version, and permission for use must always be obtained from Springer. Permissions for use may be obtained through RightsLink at the Copyright Clearance Center. Violations are liable to prosecution under the respective Copyright Law. -

Genetic Variability in the Pistachio Late Blight Fungus, Alternaria Alternata
Mycol. Res. 105 (3): 300–306 (March 2001). Printed in the United Kingdom. 300 Genetic variability in the pistachio late blight fungus, Alternaria alternata Mallikarjuna K. ARADHYA*, Helen M. CHAN and Dan E. PARFITT Department of Pomology, University of California, One Shields Avenue, Davis, CA 95616, USA. E-mail: aradhya!ucdavis.edu Received 15 December 1999; accepted 21 August 2000 Genetic variation in the pistachio late blight fungus, Alternaria alternata, was investigated by restriction fragment length polymorphism (RFLP) in the rDNA region. Southern hybridization of EcoRI, HindIII, and XbaI digested fungal DNA with a RNA probe derived from Alt1, an rDNA clone isolated from a genomic library of the Japanese pear pathotype of A. alternata, revealed 34 different rDNA haplotypes among 56 isolates collected from four central valley locations in California. Analysis of molecular variation revealed a significant amount of genetic diversity within populations (85n8%), with only marginal variation accounting for differentiation among populations (14n2%, ΦST l 0n142). All isolates examined were highly pathogenic. The identity of the four geographic populations sampled was not evident in both cluster and principal component analyses, probably indicating either the selectively neutral nature of rDNA variation or prevalence of widespread gene flow among populations combined with uniform host-selection. INTRODUCTION 1998). Biochemical and molecular markers have been used to demonstrate the existence of multiple strains and to assess The genus Alternaria contains diverse and ubiquitous species of infraspecific variation in A. alternata at the protein and DNA fungi, including aggressive and opportunistic plant pathogens level (Petrunak & Christ 1992, Adachi et al. 1993, Weir et al. -

Ascochyta Pisi, a Disease of Seed Peas
April, 1906.} Ascoehytapisi—Disease of Seed Peas. 507 ASCOCHYTA PISI,—A DISEASE OF SEED PEAS.1 J. M. VAN HOOK. During the season of 1904 and 1905, there was an exceptional blighting2 of peas from Ascochyta pisi Lib. The disease was general throughout the state and occasioned loss especially where peas are grown in large areas for canning purposes. My attention was first called to this trouble June 24, 1904, on French June field peas, which had been sown with oats as a for- age crop. Most of the peas at this time, were about two feet high and just beginning to bloom. The lower leaves were, for the most part, dead. A few plants were wilting after several days of sunshine following continuous wet weather. Other stunted peas grew among these, some of which never attained a height greater than a few inches. Appearance on stems, leaves, pods and seed.—A close examina- tion of the plants showed that the stems had been attacked at many points, frequently as high as one and one-half feet from the ground, though most severely near the ground where the disease starts. In the beginning, dead areas were formed on the stem in the form of oval or elongated lesions. At a point, from the top of the ground to two or three inches above the ground, these lesions were so numerous and had spread so rapidly as to become continuous, leaving the stem encircled by a dead area. In some cases, the woody part of the stem was also dead, though the greater number of such plants still remained green above. -

Mycosphere Notes 225–274: Types and Other Specimens of Some Genera of Ascomycota
Mycosphere 9(4): 647–754 (2018) www.mycosphere.org ISSN 2077 7019 Article Doi 10.5943/mycosphere/9/4/3 Copyright © Guizhou Academy of Agricultural Sciences Mycosphere Notes 225–274: types and other specimens of some genera of Ascomycota Doilom M1,2,3, Hyde KD2,3,6, Phookamsak R1,2,3, Dai DQ4,, Tang LZ4,14, Hongsanan S5, Chomnunti P6, Boonmee S6, Dayarathne MC6, Li WJ6, Thambugala KM6, Perera RH 6, Daranagama DA6,13, Norphanphoun C6, Konta S6, Dong W6,7, Ertz D8,9, Phillips AJL10, McKenzie EHC11, Vinit K6,7, Ariyawansa HA12, Jones EBG7, Mortimer PE2, Xu JC2,3, Promputtha I1 1 Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand 2 Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Kunming 650201, China 3 World Agro Forestry Centre, East and Central Asia, 132 Lanhei Road, Kunming 650201, Yunnan Province, People’s Republic of China 4 Center for Yunnan Plateau Biological Resources Protection and Utilization, College of Biological Resource and Food Engineering, Qujing Normal University, Qujing, Yunnan 655011, China 5 Shenzhen Key Laboratory of Microbial Genetic Engineering, College of Life Sciences and Oceanography, Shenzhen University, Shenzhen 518060, China 6 Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai 57100, Thailand 7 Department of Entomology and Plant Pathology, Faculty of Agriculture, Chiang Mai University, Chiang Mai 50200, Thailand 8 Department Research (BT), Botanic Garden Meise, Nieuwelaan 38, BE-1860 Meise, Belgium 9 Direction Générale de l'Enseignement non obligatoire et de la Recherche scientifique, Fédération Wallonie-Bruxelles, Rue A. -
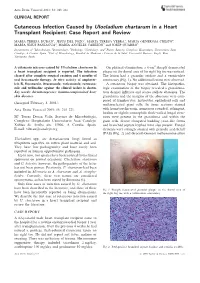
Cutaneous Infection Caused by Ulocladium Chartarum in a Heart Transplant Recipient: Case Report and Review
Acta Derm Venereol 2003; 83: 218–221 CLINICAL REPORT Cutaneous Infection Caused by Ulocladium chartarum in a Heart Transplant Recipient: Case Report and Review MARI´A TERESA DURA´ N1, JESU´ S DEL POZO2, MARI´A TERESA YEBRA3, MARI´A GENEROSA CRESPO4, MARI´A JESU´ S PANIAGUA4, MARI´A ANGELES CABEZO´ N5 and JOSEP GUARRO6 Departments of 1Microbiology, 2Dermatology, 3Pathology, 4Cardiology, and 5Plastic Surgery, Complexo Hospitalario Universitario Juan Canalejo, A Corun˜a, Spain, 6Unit of Microbiology, Facultat de Medicina i Cie`ncies de la Salut, Universitat Rovira i Virgili, Reus, Tarragona, Spain A cutaneous mycoses caused by Ulocladium chartarum in On physical examination, a 6-cm2 sharply demarcated a heart transplant recipient is reported. The infection plaque on the dorsal area of his right big toe was noticed. cleared after complete surgical excision and 6 months of The lesion had a granular surface and a vermiculate oral itraconazole therapy. In vitro activity of amphoter- consistency (Fig. 1). No additional lesions were observed. icin B, fluconazole, itraconazole, voriconazole, ravucona- A cutaneous biopsy was obtained. The histopatho- zole and terbinafine against the clinical isolate is shown. logic examination of the biopsy revealed a granuloma- Key words: dermatomycoses; immunocompromised host; tous dermal infiltrate and scarce stellate abscesses. The skin diseases. granuloma and the margins of the abscesses were com- posed of lymphocytes, histiocytes, epithelioid cells and (Accepted February 3, 2003.) multinucleated giant cells. In tissue sections stained Acta Derm Venereol 2003; 83: 218–221. with hematoxylin-eosin, numerous rounded, refringent, hyaline or slightly eosinophilic thick-walled fungal struc- Ma Teresa Dura´n Valle, Servicio de Microbiologı´a, tures were present in the granuloma and within the Complexo Hospitalario Universitario Juan Canalejo, giant cells. -

Characterization of Alternaria Alternata Isolates Causing Brown Spot of Potatoes in South Africa
Characterization of Alternaria alternata isolates causing brown spot of potatoes in South Africa By Joel Prince Dube Submitted in partial fulfilment of the requirements for the degree of Master in Science (Agriculture) Plant Pathology In the faculty of Natural and Agricultural Sciences Department of Microbiology and Plant Pathology University of Pretoria Pretoria February 2014 © University of Pretoria DECLARATION I, Joel Prince Dube, declare that the thesis, which I hereby submit for the degree Master of Science (Agriculture) Plant Pathology at the University of Pretoria, is my own work and has not been previously submitted by me for a degree at this or any other tertiary institution. Signed: ___________________________ Date: ____________________________ i © University of Pretoria Acknowledgements I would like to extend my heartfelt thanks the contributions of the following: 1. First and foremost, the Almighty God by whose grace I am where I am today. I owe everything to him. 2. My supervisors, Prof. Jacquie van der Waals and Dr. Mariette Truter, for their unwavering support and guidance throughout my Masters journey. 3. Pathology programme @ UP for the opportunity and funding for my studies. 4. Syngenta for funding one of my chapters. 5. Charles Wairuri, Nelisiwe Khumalo, Alain Misse for their help with all my molecular work. 6. Colleagues in greenhouse for all their help, suggestions and contributions throughout my studies. 7. My family and friends for their financial, spiritual and moral support, it is greatly appreciated. ii © University of Pretoria Characterization of Alternaria alternata isolates causing brown spot of potatoes in South Africa By Joel Prince Dube Supervisor : Prof. J. -

Taxonomy and Multigene Phylogenetic Evaluation of Novel Species in Boeremia and Epicoccum with New Records of Ascochyta and Didymella (Didymellaceae)
Mycosphere 8(8): 1080–1101 (2017) www.mycosphere.org ISSN 2077 7019 Article Doi 10.5943/mycosphere/8/8/9 Copyright © Guizhou Academy of Agricultural Sciences Taxonomy and multigene phylogenetic evaluation of novel species in Boeremia and Epicoccum with new records of Ascochyta and Didymella (Didymellaceae) Jayasiri SC1,2, Hyde KD2,3, Jones EBG4, Jeewon R5, Ariyawansa HA6, Bhat JD7, Camporesi E8 and Kang JC1 1 Engineering and Research Center for Southwest Bio-Pharmaceutical Resources of National Education Ministry of China, Guizhou University, Guiyang, Guizhou Province 550025, P.R. China 2Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai 57100, Thailand 3World Agro forestry Centre East and Central Asia Office, 132 Lanhei Road, Kunming 650201, P. R. China 4Botany and Microbiology Department, College of Science, King Saud University, Riyadh, 1145, Saudi Arabia 5Department of Health Sciences, Faculty of Science, University of Mauritius, Reduit, Mauritius 6Department of Plant Pathology and Microbiology, College of BioResources and Agriculture, National Taiwan University, No.1, Sec.4, Roosevelt Road, Taipei 106, Taiwan, ROC. 7No. 128/1-J, Azad Housing Society, Curca, P.O. Goa Velha, 403108, India 89A.M.B. Gruppo Micologico Forlivese “Antonio Cicognani”, Via Roma 18, Forlì, Italy; A.M.B. CircoloMicologico “Giovanni Carini”, C.P. 314, Brescia, Italy; Società per gliStudiNaturalisticidella Romagna, C.P. 144, Bagnacavallo (RA), Italy *Correspondence: [email protected] Jayasiri SC, Hyde KD, Jones EBG, Jeewon R, Ariyawansa HA, Bhat JD, Camporesi E, Kang JC 2017 – Taxonomy and multigene phylogenetic evaluation of novel species in Boeremia and Epicoccum with new records of Ascochyta and Didymella (Didymellaceae).