Flame Retardant Functional Textiles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Appendix 1 Sources
APPENDIX 1 SOURCES UMIST: DEPARTMENT OF TEXTILES Most of the work described in this book comes from research in the Department of Textiles, UMIST, under the direction of Professor John Hearle. It started with the purchase of a scanning electron microscope with a grant from the Science Research Council in 1967, together with five-year funding for an experimental officer and a technician. Since 1972, the staff have been supported by general UMIST funds; a second grant from SERC enabled a replacement SEM to be bought in 1979; industrial sponsors, listed below, have contributed through membership of the Fibre Fracture Research Group; special research grants have been made by the Ministry of Defence (SCRDE, Colchester, and RAE, Farnborough) and jointly by the Wool Research Organization of New Zealand (WRONZ) and the Wool Foundation (IWS); and other research programmes and contract services have contributed indirectly to our knowledge. Pat Cross was the first SEM experimental officer and she was followed in 1969 by Brenda Lomas, who retired in 1990. Trevor Jones then took on responsibility for microscopy in the Department of Textiles in addition to photography. Over the years, many staff and students have contributed to the research. Their names are given below. Some have worked wholly on fibre fracture problems. Others have used fracture studies as an incidental element in their work. PERSONNEL The following people at UMIST have contributed to the research. Academic staff J.D. Berry Aspects of fibre breakage CP. Buckley Mechanics of tensile fracture, general direction C. Carr Fabric studies W.D. Cooke Pilling in knitwear, conservation studies G.E. -

Comprehensive Rope Catalog
2019-2020 Comprehensive Rope Catalog pelicanrope.com Offering a wide variety of tools and hardware: DURABRITE™ Rigging Hardware YOKE Lifting ProClimb™ Fall Protection / Climbing FEENEY ARCHITECTURAL RAILING DURABRITE™ Stainless Hardware SwageRight™ Tools Uncompromising service for more than 40 years Call Today for a Free Quote! (888) 260 - 7444 Are you a distributor? Contact: [email protected] for details. www.usrigging.com www.usriggingdepot.com Climbing & Rescue Rope 06-11 Static Kernmantle Life Lines Fire & Escape Rope Water Rescue Rope Arborist Climbing Line & Bull Rope Prusik Lines & Accessory Cords of high Industrial, Utility & Marine Rope 12-24 Pelican Rope is an ISO 9001:2015 certified manufacturer performance synthetic and specialty rope products, rope lanyards, slings and custom rope assemblies. For over 40 years our devotion 12 Strand & Krypton Rope Cable Pulling Rope to innovation, quality and customer service has yielded a diverse Double Braid UL Certified product line designed to not only meet or exceed the standards established by the Cordage Institute, the U.S. Military, ASTM and NFPA but more importantly, the expectations of our loyal customers in the commercial, industrial, arborist, fire and Assemblies & Lanyards 25-36 rescue, law enforcement, manufacturing and marine industries. Dependability is our hallmark; exceptional value our everyday Flip Lines standard. Split Tails Lanyards & Runners Vertical Life Lines Dead Eye Slings American Made Matters Anchor & Dock Lines Boom Truck Winch Lines www.pelicanrope.com General Cordage 37-41 Call Us Toll Free: (800) 464-7673 Spun Polyester Corporate Office, Factory & Warehouse: Solid Braid 1600 E. McFadden Avenue, Santa Ana, CA 92705 Hollow Braid 3 Strand Rope Wire Core Rope Rope & Fall Protection Accessories 42-45 Chafe Gear Shrink Tube Rope Accessories Terminations ISO 9001:2015 CERTIFIED All rope should be inspected before and after every use for any form of damage. -

Saatibelt Fabrics for Conveyor and Dryer Belts SAATI Group and Its Filtration Applications
SAATIbelt Fabrics for Conveyor and Dryer Belts SAATI Group and its Filtration Applications SAATI is a multinational group with corporate The products are used in a wide range of headquarters situated in northern Italy since different filtration fields such as, automotive, 1935. Today we are a leader in the development, water, healthcare, app consumer electronics, manufacturing and commercialization of advanced food & milling, along with many other industrial technical textiles and chemicals. applications. SAATI specializes in precise woven fabrics and SAATIbelt® fabrics are constructed from chemical technologies used in the fields of technically advanced fibers, which offer the best Filtration, Screen printing, Structural composites in resistance, reliability and life. and Ballistic protection. The development of They are used in an extensive variety of enhanced performance coatings lies at SAATI’s applications, including textile, tannery, ceramics, core. screen-printing, packaging, transportation, SAATI - Filtration specializes in the production lamination and food processing. of technical precision (with monofilament and multifilament yarn) fabrics and components in polyamide, polyester and polypropylene, with special finishing treatments. Focuses on Customer and Innovation Thanks to our direct presence in many countries, At SAATI, we have a real attitude for innovation it is easy for the customers to reach us, wherever and to a continuous research of processes they are located, and our responsiveness is always and materials that make real improvements in prompt. Our staff has a high level of technical production and service. SAATI acts in the market expertise and dedication, always aiming to finding with this attitude, offering sieving and filter the best solution for the customer’s requirements. -

Temperature and Humidity Aging of Poly(P-Phenylene-2,6
Polymer Degradation and Stability 92 (2007) 1234e1246 www.elsevier.com/locate/polydegstab Temperature and humidity aging of poly( p-phenylene-2,6- benzobisoxazole) fibers: Chemical and physical characterization Joannie Chin a,*, Amanda Forster b, Cyril Clerici a, Lipiin Sung a, Mounira Oudina a, Kirk Rice b a Polymeric Materials Group, National Institute of Standards and Technology, Gaithersburg, MD 20899, United States b Office of Law Enforcement Standards, National Institute of Standards and Technology, Gaithersburg, MD 20899, United States Received 9 February 2007; received in revised form 20 March 2007; accepted 23 March 2007 Available online 19 April 2007 Abstract In recent years, poly( p-phenylene-2,6-benzobisoxazole) (PBO) fibers have become prominent in high strength applications such as body ar- mor, ropes and cables, and recreational equipment. The objectives of this study were to expose woven PBO body armor panels to elevated tem- perature and moisture, and to analyze the chemical, morphological and mechanical changes in PBO yarns extracted from the panels. A 30% decrease in yarn tensile strength, which was correlated to changes in the infrared peak absorbance of key functional groups in the PBO structure, was observed during the 26 week elevated temperature/elevated moisture aging period. Substantial changes in chemical structure were observed via infrared spectroscopy, as well as changes in polymer morphology using microscopy and neutron scattering. When the panels were removed to an ultra-dry environment for storage for 47 weeks, no further decreases in tensile strength degradation were observed. In a follow-on study, fibers were sealed in argon-filled glass tubes and exposed to elevated temperature; less than a 4% decrease in tensile strength was observed after 30 weeks, demonstrating that moisture is a key factor in the degradation of these fibers. -

Wire Harness Yarn Fibers Processes Products
MOVING HIGH PERFORMANCE FIBERS FORWARD WIRE HARNESS YARN FIBERS PROCESSES PRODUCTS WHY FIBER-LINE® WIRE HARNESS YARN? Key Features Overview • Available flat, twisted, or cord • FIBER-LINE® provides a wide range of products utilized for wire harnesses. • Reduction in overall weight & energy usage As demand for electrical and electronic content is growing in industries • High temperature resistance such as automotive, aerospace, and robotics, wire harness designs are • Protection from thermal degradation more complex and their performance requirements are increasing. • Various colors available for easy identification • FIBER-LINE® wire harness yarn allows you to produce thinner, lighter, and • Abrasion resistant more durable products. Since our yarn are lighter and thinner than metal, you can conserve more valuable space and provide greater design flexibility. FIBER-LINE® FIBERS • FIBER-LINE® high-performance fibers protect against thermal de-gradation, FOR WIRE HARNESS YARN resist harsh fluids, and add insulation. • Kevlar® Para-Aramid • FIBER-LINE® Colorcoat™ replaces recently discontinued solution-dyed • Nomex® Meta-Aramid products. • Technora® Para-Aramid • PET Polyester Packaging FIBER-LINE® Wire Harness Yarn are supplied on a variety of cardboard tubes FIBER-LINE® PERFORMANCE to meet your equipment needs. Contact us today with tube dimensions ADDING COATINGS you require. Packages can be supplied on colored, embossed and/or slit tubes. • FIBER-LINE® Colorcoat™: Plastic or metal reels are also available. Color Identification • FIBER-LINE® -

Pegasus Ropes Brochure
STRENGTH ™ that DEFIES GRAVITY ® ® PRODUCTSWITH A MISSION North American Rescue® Pegasus Ropes® | 888.689.6277 | www.narescue.com 1 Redefining Rope Technologies Understanding Ropes vs Environment North American Rescue (NAR) is proud to announce our Static Pegasus Ropes® Series – the lightest weight and most versatile rope available today. Understanding your mission requirements, NAR ® continues our tradition of providing tactical and rescue teams with innovative and dependable equipment that outperforms expectations in unpredictable environments. PEGASUS cores are all designed with Innegra™S, Our Pegasus Ropes® line delivers unmatched performance a new and innovative advanced fiber (wet, dry or contaminated), allowing you to utilize one rope that gives Pegasus Ropes® the same physical & for most missions. mechanical properties – whether wet, dry or even Pegasus cores are composed of 100% virgin Innegra™ fiber. exposed to chemicals. This breakthrough Innegra™ is a new, advanced material with excellent physical technology has an impressive strength-to-weight & mechanical properties. Characterized by its excellent ratio, making it the lightest structural fiber available strength to weight ratio, Innegra™S delivers a today. Innegra™’s unique composition also provides very strong, ultra-lightweight, hydrophobic, buoyant great thermal properties when used and chemically inert fiber that is ideal for varying in hot or cold environments. For the operational needs. first time you can be confident that the backbone of your rope’s system, Unlike all nylon based ropes, Pegasus’ physical and your core, will perform mechanical properties are virtually unchanged regardless regardless of environmental of environmental conditions such as rain, sleet, snow, challenges. mist, humidity, salt water and chemicals, allowing it to maintain its high strength, low weight and cut resistance HARNESSING THE ADVANTAGE OF regardless of the weather. -
All About Fibers
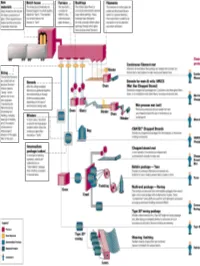
RawRaw MaterialsMaterials ¾ More than half the mix is silica sand, the basic building block of any glass. ¾ Other ingredients are borates and trace amounts of specialty chemicals. Return © 2003, P. Joyce BatchBatch HouseHouse && FurnaceFurnace ¾ The materials are blended together in a bulk quantity, called the "batch." ¾ The blended mix is then fed into the furnace or "tank." ¾ The temperature is so high that the sand and other ingredients dissolve into molten glass. Return © 2003, P. Joyce BushingsBushings ¾The molten glass flows to numerous high heat-resistant platinum trays which have thousands of small, precisely drilled tubular openings, called "bushings." Return © 2003, P. Joyce FilamentsFilaments ¾This thin stream of molten glass is pulled and attenuated (drawn down) to a precise diameter, then quenched or cooled by air and water to fix this diameter and create a filament. Return © 2003, P. Joyce SizingSizing ¾The hair-like filaments are coated with an aqueous chemical mixture called a "sizing," which serves two main purposes: 1) protecting the filaments from each other during processing and handling, and 2) ensuring good adhesion of the glass fiber to the resin. Return © 2003, P. Joyce WindersWinders ¾ In most cases, the strand is wound onto high-speed winders which collect the continuous fiber glass into balls or "doffs.“ Single end roving ¾ Most of these packages are shipped directly to customers for such processes as pultrusion and filament winding. ¾ Doffs are heated in an oven to dry the chemical sizing. Return © 2003, P. Joyce IntermediateIntermediate PackagePackage ¾ In one type of winding operation, strands are collected into an "intermediate" package that is further processed in one of several ways. -

The World of Teijin Aramid
Teijin Aramid @ Techtextile Middle East Symposium Dubai , 20th Feburary 2014 René Lohmann Sales & Marketing Ballistics Teijin Aramid GmbH, Wuppertal, Germany Agenda • Global Key Trends • Aramids in the middle East • Stopping the bullet • 550dtex f1000 ballistic yarn • LFT SB1 Plus • SRM • Microflex • Twaron and Endumax in helmets • Our research capabilities • Sustainable strength Global Key Trends Global key trends • In recent years, there have been significant changes in the requirements placed on both consumer and industrial goods around the world • There is a growing demand for products that combine high performance with durability and low maintenance • At the same time, these products need to be cost-effective, use less energy, enhance safety, and they should ideally have a smaller lifecycle ecological footprint Sharing our customers’ ambitions • Our prime aim is to add value to the bottom line of our customers • Co-creation and open innovation with customers on advanced products and applications • Loyalty to customers • Long-term relationships • Sharing knowledge & expertise Global presence Aramid in the middle East Our product portfolio Para-aramid • Twaron • Technora Meta-aramid • Teijinconex Poly-ethylene • Endumax Different types to fit application requirements Twaron Technora Short-cut fiber Staple fiber Pulp Fabrics Tape Powder Short-cut fibers Endumax , UHMWPE Tape and X-ply • Ropes, cables and slings • Ballistic protection • Robotics / Force transmission Technora, for enhanced properties • High tensile strength • Weight for -

Cryogenic Temperature Effects on the Mechanical Properties
CRYOGENIC TEMPERATURE EFFECTS ON THE MECHANICAL PROPERTIES OF CARBON, ARAMID, AND PBO FIBERS By William Chad Hastings A Thesis Submitted to the Faculty of Mississippi State University in Partial Fulfillment of the Requirements for the Degree of Master of Science in Mechanical Engineering in the Department of Mechanical Engineering Mississippi State, Mississippi May 2008 CRYOGENIC TEMPERATURE EFFECTS ON THE MECHANICAL PROPERTIES OF CARBON, ARAMID, AND PBO FIBERS By William Chad Hastings Approved: ______________________________ ______________________________ Judy A. Schneider Anthony J. Vizzini Associate Professor of Mechanical Professor and Head of Aerospace Engineering Engineering Department (Director of Thesis) (Committee Member) ______________________________ ______________________________ Thomas E. Lacy Steve Daniewicz Associate Professor of Aerospace Professor and Graduate Coordinator Engineering of Mechanical Engineering (Committee Member) Department ______________________________ W.G. Steele Interim Dean and Professor Bagley College of Engineering Name: William Chad Hastings Date of Degree: May 2, 2008 Institution: Mississippi State University Major Field: Mechanical Engineering Major Professor: Judy Schneider Title of Study: CRYOGENIC TEMPERATURE EFFECTS ON THE MECHANICAL PROPERTIES OF CARBON, ARAMID, AND PBO FIBERS Pages in Study: 39 Candidate for Degree of Master of Science This study examines the effects of cryogenic temperatures on the mechanical properties of carbon, aramid, and poly(p-phenylene-2, 6-benzobisoxazole) (PBO) fibers. Although the mechanical properties are documented for these fibers at ambient and elevated temperatures, there is an absence of data in the open literature for how these fibers behave at very low temperatures. To evaluate the mechanical properties, the ASTM standard method for testing at ambient temperature was used as a baseline. The low temperature tests were conducted inside a double walled cryogenic chamber to evaluate the fiber performance at 100K. -

Work 2018 Our Training Center
Arbor Fire & Rescue Tower Rope Access Tactical Work 2018 Our Training Center For training companies and organizations who need to teach or certify employees in rope-rescue techniques, the training center is a valuable resource. Key features in the simulation training space are a free-standing 30-ft. radio tower, a vertical wall with removable window and door plugs for bailout training, and anchors for practicing edge, raise and rescue, and hauling operations. Conveniently located in Biddeford, Maine, the Training Center is 20 minutes south of the Portland Jetport, with easy access to Portland’s scenic coastline, downtown shopping, and renowned restaurant scene. To schedule your next training at the Sterling Training Center, contact Matt Hunt at 1-207-282-3107 or Alex Hebbel training on the FCX Descent Control Device. [email protected]. Sterling’s new state-of-the art training center is a testament to their commitment towards improving safe practices within work-at-height trades. Equipped with ample lighting, abundant seating, and vertical structural supports fully rated up to 5,000 pounds, the center is the ideal learning environment for rope access, arboricultural, fire/ Matt Hunt demonstrating escape kits and systems to a fire department. rescue, tower, and other work-at-height trainings. The center is located within the Sterling factory, allowing guests the opportunity to observe the full life cycle of a product from its raw material state to production to testing and, finally, to quality assurance. Few companies have made such a viable investment in the industries they serve, proving Sterling not only believes in producing a high- quality product, but providing the training environment for customers to learn how to use the product safely. -

(Pbo) Fibers Under Elevated Temperature and Humidi
FACTORS CONTRIBUTING TO THE DEGRADATION OF POLY(P- PHENYLENE BENZOBISOXAZOLE) (PBO) FIBERS UNDER ELEVATED TEMPERATURE AND HUMIDITY CONDITIONS A Thesis by JOSEPH M. O’NEIL Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE August 2006 Major Subject: Mechanical Engineering FACTORS CONTRIBUTING TO THE DEGRADATION OF POLY(P- PHENYLENE BENZOBISOXAZOLE) (PBO) FIBERS UNDER ELEVATED TEMPERATURE AND HUMIDITY CONDITIONS A Thesis by JOSEPH M. O’NEIL Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Approved by: Chair of Committee, Roger Morgan Committee Members, Jaime Grunlan Michael Bevan Head of Department, Dennis O’Neal August 2006 Major Subject: Mechanical Engineering iii ABSTRACT Factors Contributing to the Degradation of Poly(p-phenylene benzobisoxazole) (PBO) Fibers under Elevated Temperature and Humidity Conditions. (August 2006) Joseph M. O’Neil, B.S., Texas A&M University Chair of Advisory Committee: Dr. Roger J. Morgan The moisture absorption behavior of Zylon fibers was characterized in various high temperature and high humidity conditions in a controlled environment. The results of these thermal cycling tests show that PBO fibers not only absorb, but also retain moisture (approximately 0.5-3%) when exposed to elevated temperature and humidity cycles. Also, the impurities of Zylon fibers were characterized through the use of Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS) and solid state Nuclear Magnetic Resonance (NMR). These tests demonstrated that, in addition to other impurities, PBO fibers may contain up to 0.55 weight percent phosphorus, and that this phosphorus is present in the form of phosphoric acid. -

Ballistic Impact Response of Kevlar 49 and Zylon Under Conditions Representing Jet Engine Fan Containment
Ballistic Impact Response of Kevlar 49 and Zylon Under Conditions Representing Jet Engine Fan Containment J. Michael Pereira and Duane M. Revilock NASA Glenn Research Center, Cleveland, Ohio [email protected] [email protected] Abstract A ballistic impact test program was conducted to provide validation data for the development of numerical models of blade out events in fabric containment systems. The impact response of two different fiber materials - Kevlar 49 (E.I. DuPont Nemours and Company) and Zylon AS (Toyobo Co., Ltd.) was studied by firing metal projectiles into dry woven fabric specimens using a gas gun. The shape, mass, orientation and velocity of the projectile were varied and recorded. In most cases the tests were designed such that the projectile would perforate the specimen, allowing measurement of the energy absorbed by the fabric. The results for both Zylon and Kevlar presented here represent a useful set of data for the purposes of establishing and validating numerical models for predicting the response of fabrics under conditions simulating those of a jet engine blade release situation. In addition some useful empirical observations were made regarding the effects of projectile orientation and the relative performance of the different materials. Introduction In the last thirty years the use of aramid fabrics in jet engine blade containment systems has become common. It is recognized that high strength and high elongation fabrics, combined with innovative structural concepts can provide a light weight, effective fan case system that provides the strength required to safely handle impact loads, blade rub loads and the large dynamic loads caused by rotor imbalance.