Observations of Recurrent Laryngeal Nerve Injury and Recovery Using a Rat Model
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neuroanatomy Crash Course
Neuroanatomy Crash Course Jens Vikse ∙ Bendik Myhre ∙ Danielle Mellis Nilsson ∙ Karoline Hanevik Illustrated by: Peder Olai Skjeflo Holman Second edition October 2015 The autonomic nervous system ● Division of the autonomic nervous system …………....……………………………..………….…………... 2 ● Effects of parasympathetic and sympathetic stimulation…………………………...……...……………….. 2 ● Parasympathetic ganglia ……………………………………………………………...…………....………….. 4 Cranial nerves ● Cranial nerve reflexes ………………………………………………………………….…………..…………... 7 ● Olfactory nerve (CN I) ………………………………………………………………….…………..…………... 7 ● Optic nerve (CN II) ……………………………………………………………………..…………...………….. 7 ● Pupillary light reflex …………………………………………………………………….…………...………….. 7 ● Visual field defects ……………………………………………...................................…………..………….. 8 ● Eye dynamics …………………………………………………………………………...…………...………….. 8 ● Oculomotor nerve (CN III) ……………………………………………………………...…………..………….. 9 ● Trochlear nerve (CN IV) ………………………………………………………………..…………..………….. 9 ● Trigeminal nerve (CN V) ……………………………………………………................…………..………….. 9 ● Abducens nerve (CN VI) ………………………………………………………………..…………..………….. 9 ● Facial nerve (CN VII) …………………………………………………………………...…………..………….. 10 ● Vestibulocochlear nerve (CN VIII) …………………………………………………….…………...…………. 10 ● Glossopharyngeal nerve (CN IX) …………………………………………….……….…………...………….. 10 ● Vagus nerve (CN X) …………………………………………………………..………..…………...………….. 10 ● Accessory nerve (CN XI) ……………………………………………………...………..…………..………….. 11 ● Hypoglossal nerve (CN XII) …………………………………………………..………..…………...…………. -

Structural Brain Abnormalities in Patients with Primary Open-Angle Glaucoma: a Study with 3T MR Imaging
Glaucoma Structural Brain Abnormalities in Patients with Primary Open-Angle Glaucoma: A Study with 3T MR Imaging Wei W. Chen,1–3 Ningli Wang,1,3 Suping Cai,3,4 Zhijia Fang,5 Man Yu,2 Qizhu Wu,5 Li Tang,2 Bo Guo,2 Yuliang Feng,2 Jost B. Jonas,6 Xiaoming Chen,2 Xuyang Liu,3,4 and Qiyong Gong5 PURPOSE. We examined changes of the central nervous system CONCLUSIONS. In patients with POAG, three-dimensional MRI in patients with advanced primary open-angle glaucoma revealed widespread abnormalities in the central nervous (POAG). system beyond the visual cortex. (Invest Ophthalmol Vis Sci. 2013;54:545–554) DOI:10.1167/iovs.12-9893 METHODS. The clinical observational study included 15 patients with bilateral advanced POAG and 15 healthy normal control subjects, matched for age and sex with the study group. Retinal rimary open angle glaucoma (POAG) has been defined nerve fiber layer (RNFL) thickness was measured by optical formerly by intraocular morphologic changes, such as coherence tomography (OCT). Using a 3-dimensional magne- P progressive retinal ganglion cell loss and defects in the retinal tization-prepared rapid gradient-echo sequence (3D–MP-RAGE) nerve fiber layer (RNFL), and by corresponding psychophysical of magnetic resonance imaging (MRI) and optimized voxel- abnormalities, such as visual field loss.1 Recent studies by based morphometry (VBM), we measured the cross-sectional various researchers, however, have suggested that the entire area of the optic nerve and optic chiasm, and the gray matter visual pathway may be involved in glaucoma.2–23 Findings from volume of the brain. -

Asymmetries of Dark and Bright Negative Afterimages Are Paralleled by Subcortical on and OFF Poststimulus Responses
1984 • The Journal of Neuroscience, February 22, 2017 • 37(8):1984–1996 Systems/Circuits Asymmetries of Dark and Bright Negative Afterimages Are Paralleled by Subcortical ON and OFF Poststimulus Responses X Hui Li,1,2 X Xu Liu,1,2 X Ian M. Andolina,1 Xiaohong Li,1 Yiliang Lu,1 Lothar Spillmann,3 and Wei Wang1 1Institute of Neuroscience, State Key Laboratory of Neuroscience, Key Laboratory of Primate Neurobiology, Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai 200031, China, 2University of Chinese Academy of Sciences, Shanghai 200031, China, and 3Department of Neurology, University of Freiburg, 79085 Freiburg, Germany Humans are more sensitive to luminance decrements than increments, as evidenced by lower thresholds and shorter latencies for dark stimuli. This asymmetry is consistent with results of neurophysiological recordings in dorsal lateral geniculate nucleus (dLGN) and primary visual cortex (V1) of cat and monkey. Specifically, V1 population responses demonstrate that darks elicit higher levels of activation than brights, and the latency of OFF responses in dLGN and V1 is shorter than that of ON responses. The removal of a dark or bright disc often generates the perception of a negative afterimage, and here we ask whether there also exist asymmetries for negative afterimages elicited by dark and bright discs. If so, do the poststimulus responses of subcortical ON and OFF cells parallel such afterimage asymmetries? To test these hypotheses, we performed psychophysical experiments in humans and single-cell/S-potential recordings in cat dLGN. Psychophysically, we found that bright afterimages elicited by luminance decrements are stronger and last longer than dark afterimages elicited by luminance increments of equal sizes. -

Embryology, Anatomy, and Physiology of the Afferent Visual Pathway
CHAPTER 1 Embryology, Anatomy, and Physiology of the Afferent Visual Pathway Joseph F. Rizzo III RETINA Physiology Embryology of the Eye and Retina Blood Supply Basic Anatomy and Physiology POSTGENICULATE VISUAL SENSORY PATHWAYS Overview of Retinal Outflow: Parallel Pathways Embryology OPTIC NERVE Anatomy of the Optic Radiations Embryology Blood Supply General Anatomy CORTICAL VISUAL AREAS Optic Nerve Blood Supply Cortical Area V1 Optic Nerve Sheaths Cortical Area V2 Optic Nerve Axons Cortical Areas V3 and V3A OPTIC CHIASM Dorsal and Ventral Visual Streams Embryology Cortical Area V5 Gross Anatomy of the Chiasm and Perichiasmal Region Cortical Area V4 Organization of Nerve Fibers within the Optic Chiasm Area TE Blood Supply Cortical Area V6 OPTIC TRACT OTHER CEREBRAL AREASCONTRIBUTING TO VISUAL LATERAL GENICULATE NUCLEUSPERCEPTION Anatomic and Functional Organization The brain devotes more cells and connections to vision lular, magnocellular, and koniocellular pathways—each of than any other sense or motor function. This chapter presents which contributes to visual processing at the primary visual an overview of the development, anatomy, and physiology cortex. Beyond the primary visual cortex, two streams of of this extremely complex but fascinating system. Of neces- information flow develop: the dorsal stream, primarily for sity, the subject matter is greatly abridged, although special detection of where objects are and for motion perception, attention is given to principles that relate to clinical neuro- and the ventral stream, primarily for detection of what ophthalmology. objects are (including their color, depth, and form). At Light initiates a cascade of cellular responses in the retina every level of the visual system, however, information that begins as a slow, graded response of the photoreceptors among these ‘‘parallel’’ pathways is shared by intercellular, and transforms into a volley of coordinated action potentials thalamic-cortical, and intercortical connections. -

Eye Essentials 5
continuing education 33 Eye essentials 5 Successful participation in each Classification and localisation module of this approved series counts as one credit towards the GOC CET scheme administered by Vantage and of visual field defects one towards the AOI’s scheme. In the last of our features based on the Eye Essential textbooks, Dr Robert Cubbidge describes the visual pathway and its relationship with the visual field. CET module C2354 This article has been adapted and abridged from Visual Fields by Dr THE DIMENSION of the blind spot Robert Cubbidge, is approximately 7.5º high and 5.5º wide part of the new and represents the temporal visual field Eye Essentials projection of the optic nerve, found series. For further approximately 1.5º below and 15º horizon- information, tally from fixation. When interpreting including ordering, please click on visual field defects, knowledge of the the Bookstore link arrangement of nerve fibres in the visual at www.optician pathway is essential. online.net Depending on the site of damage in the visual pathway, characteristic visual field defects are produced (Figure 1). course to the optic nerve as they are not Anatomically, the visual pathway hindered by the papillomacular bundle begins at the photoreceptors which lie in (Figure 2). The nerve fibres from the nasal the outer retina. Here, photons of light are retina do not cross those of the temporal absorbed by the photopigments, which are retina and thereby form a theoretical sensitive to specific regions of the visible vertical line of demarcation which passes electromagnetic spectrum. Light energy through the centre of the fovea. -

Anatomy and Physiology of the Afferent Visual System
Handbook of Clinical Neurology, Vol. 102 (3rd series) Neuro-ophthalmology C. Kennard and R.J. Leigh, Editors # 2011 Elsevier B.V. All rights reserved Chapter 1 Anatomy and physiology of the afferent visual system SASHANK PRASAD 1* AND STEVEN L. GALETTA 2 1Division of Neuro-ophthalmology, Department of Neurology, Brigham and Womens Hospital, Harvard Medical School, Boston, MA, USA 2Neuro-ophthalmology Division, Department of Neurology, Hospital of the University of Pennsylvania, Philadelphia, PA, USA INTRODUCTION light without distortion (Maurice, 1970). The tear–air interface and cornea contribute more to the focusing Visual processing poses an enormous computational of light than the lens does; unlike the lens, however, the challenge for the brain, which has evolved highly focusing power of the cornea is fixed. The ciliary mus- organized and efficient neural systems to meet these cles dynamically adjust the shape of the lens in order demands. In primates, approximately 55% of the cortex to focus light optimally from varying distances upon is specialized for visual processing (compared to 3% for the retina (accommodation). The total amount of light auditory processing and 11% for somatosensory pro- reaching the retina is controlled by regulation of the cessing) (Felleman and Van Essen, 1991). Over the past pupil aperture. Ultimately, the visual image becomes several decades there has been an explosion in scientific projected upside-down and backwards on to the retina understanding of these complex pathways and net- (Fishman, 1973). works. Detailed knowledge of the anatomy of the visual The majority of the blood supply to structures of the system, in combination with skilled examination, allows eye arrives via the ophthalmic artery, which is the first precise localization of neuropathological processes. -

Cranial Nerves II, III, IV & VI (Optic, Oculomotor, Trochlear, & Abducens)
Cranial Nerves II, III, IV & VI (Optic, Oculomotor, Trochlear, & Abducens) Lecture (13) ▪ Important ▪ Doctors Notes Please check our Editing File ▪ Notes/Extra explanation ه هذا العمل مب ين بشكل أسا يس عىل عمل دفعة 436 مع المراجعة { َوَم نْ يَ َت َو َ ّكْ عَ َلْ ا َّْلل فَهُ َوْ َحْ سْ ُ ُُْ} والتدقيق وإضافة المﻻحظات وﻻ يغ ين عن المصدر اﻷسا يس للمذاكرة ▪ Objectives At the end of the lecture, students should be able to: ✓ List the cranial nuclei related to occulomotor, trochlear, and abducent nerves in the brain stem. ✓ Describe the type and site of each nucleus. ✓ Describe the site of emergence and course of these 3 nerves. ✓ Describe the important relations of oculomotor, trochlear, and abducent nerves in the orbit ✓ List the orbital muscles supplied by each of these 3 nerves. ✓ Describe the effect of lesion of each of these 3 nerves. ✓ Describe the optic nerve and visual pathway. Recall the how these nerves exit from the brain stem: Optic (does not exit from brain stem) Occulomotor: ventral midbrain (medial aspect of crus cerebri) Trochlear: dorsal midbrain (caudal to inferior colliculus) Abducent: ventral Pons (junction b/w pons & pyramid) Brain (Ventral view) Brain stem (Lateral view) Extra-Ocular Muscles 7 muscles: (ترفع جفن العين) .Levator palpebrae superioris 1- Origin: from the roof of the orbit (4) Recti muscles: *Rectus: ماشي على ( Superior rectus (upward and medially 2- الصراط (Inferior rectus (downward and medially 3- المستقيم 4- Medial rectus (medial) (medial) 5- Lateral rectus (lateral) How to remember the 2 فحركته muscles not supplied by نفس اسمه -اسمها عكس وظيفتها- :Oblique muscles (2) 6- Superior oblique (downward and laterally) Oblique: CN3? Superior oblique goes -1 منحرفOrigin: from the roof of the orbit 7- Inferior oblique (upward and laterally) up (superior) and turns around (oblique) a notch يمشي Origin: from the anterior floor or pulley and its supply is عكس كﻻمه NB. -

Diseases of the Vitreous, Retina and Optic Nerve
Diseases of the Vitreous, Retina and Optic Nerve University of Florida Normal dog fundic appearance tapetum- reflective area of the superior X fundus optic disk retinal vessels nontapetum Cat Dog Tapetal fundus color dependent on age, breed and coat color blue until 6 to 10 wks 4, 8, 13, 18 wks Tapetal fundus cellular layer of the choroid variable boundary with the nontapetum area centralis (cone rich) – visual streak: RGCs no melanin in tapetal RPE nontapetal color depends on the degree of RPE and iris pigmentation choroidal vessels (orange) may be visible Retinal vasculature usually 3 or 4 major venules – form a circle (not always complete) on the optic disk surface up to 20 arterioles – may be tortuous Optic disk variable amount of myelin pale pink in color physiological pit ± pigmented ring Normal fundic variations Cat – circular optic disk lacks myelin – 3 major venules leave the disk edge with 3 major arterioles – Tapetum is usually yellow or green in color Normal fundic variations horse – 30-60 small blood vessels extend a short distance from the disk edge – oval optic disk – Stars of Winslow – fibrous tapetum Vitreal opacities Vitreal degeneration from inflammation, trauma, senile changes may predispose to retinal detachment leukocytes hemorrhage – resolution over months asteroid hyalosis – calcium-lipid complexes Choroidal coloboma Equatorial staphyloma: Australian Shepherds Progressive Retinal Atrophy (PRA) in the dog inherited retinal photoreceptor dysplasia or degeneration PRA: progressive loss of night -

Cranial Nerve Disorders: Clinical Manifestations and Topographyଝ
Radiología. 2019;61(2):99---123 www.elsevier.es/rx UPDATE IN RADIOLOGY Cranial nerve disorders: Clinical manifestations and topographyଝ a,∗ a b c M. Jorquera Moya , S. Merino Menéndez , J. Porta Etessam , J. Escribano Vera , a M. Yus Fuertes a Sección de Neurorradiología, Hospital Clínico San Carlos, Madrid, Spain b Servicio de Neurología, Hospital Clínico San Carlos, Madrid, Spain c Neurorradiología, Hospital Ruber Internacional, Madrid, Spain Received 17 November 2017; accepted 27 September 2018 KEYWORDS Abstract The detection of pathological conditions related to the twelve cranial pairs rep- Cranial pairs; resents a significant challenge for both clinicians and radiologists; imaging techniques are Cranial nerves; fundamental for the management of many patients with these conditions. In addition to knowl- Cranial neuropathies; edge about the anatomy and pathological entities that can potentially affect the cranial pairs, Neuralgia; the imaging evaluation of patients with possible cranial pair disorders requires specific exami- Cranial nerve palsy nation protocols, acquisition techniques, and image processing. This article provides a review of the most common symptoms and syndromes related with the cranial pairs that might require imaging tests, together with a brief overview of the anatomy, the most common underlying processes, and the most appropriate imaging tests for different indications. © 2018 SERAM. Published by Elsevier Espana,˜ S.L.U. All rights reserved. PALABRAS CLAVE Sintomatología derivada de los pares craneales: Clínica y topografía Pares craneales; Resumen La detección de la patología relacionada con los doce pares craneales representa Nervios craneales; un importante desafío, tanto para los clínicos como para los radiólogos. Las técnicas de imagen Neuropatía de pares craneales; son fundamentales para el manejo de muchos de los pacientes. -

Clinical Anatomy of the Cranial Nerves Clinical Anatomy of the Cranial Nerves
Clinical Anatomy of the Cranial Nerves Clinical Anatomy of the Cranial Nerves Paul Rea AMSTERDAM • BOSTON • HEIDELBERG • LONDON NEW YORK • OXFORD • PARIS • SAN DIEGO SAN FRANCISCO • SINGAPORE • SYDNEY • TOKYO Academic Press is an imprint of Elsevier Academic Press is an imprint of Elsevier 32 Jamestown Road, London NW1 7BY, UK The Boulevard, Langford Lane, Kidlington, Oxford OX5 1GB, UK Radarweg 29, PO Box 211, 1000 AE Amsterdam, The Netherlands 225 Wyman Street, Waltham, MA 02451, USA 525 B Street, Suite 1800, San Diego, CA 92101-4495, USA First published 2014 Copyright r 2014 Elsevier Inc. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. Details on how to seek permission, further information about the Publisher’s permissions policies and our arrangement with organizations such as the Copyright Clearance Center and the Copyright Licensing Agency, can be found at our website: www.elsevier.com/permissions. This book and the individual contributions contained in it are protected under copyright by the Publisher (other than as may be noted herein). Notices Knowledge and best practice in this field are constantly changing. As new research and experience broaden our understanding, changes in research methods, professional practices, or medical treatment may become necessary. Practitioners and researchers must always rely on their own experience and knowledge in evaluating and using any information, methods, compounds, or experiments described herein. In using such information or methods they should be mindful of their own safety and the safety of others, including parties for whom they have a professional responsibility. -
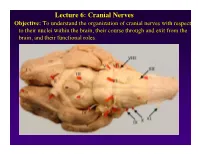
Lecture 6: Cranial Nerves
Lecture 6: Cranial Nerves Objective: To understand the organization of cranial nerves with respect to their nuclei within the brain, their course through and exit from the brain, and their functional roles. Olfactory Eye Muscles 3, 4 &6 Cranial Nerves 1-7 I overview Table, Page 49 II Lecture notes Cranial Nerves and their Functions V Trigeminal VII Facial VIII IX X XII XI Cranial Nerves 8-12 Overview sternocephalic I. Factors Responsible for the Complex Internal Organization of the Brain Stem-> leads to altered location of cranial nerve nuclei in adult brain stem 1. Development of the Fourth Ventricle a. Medulla and Pons develop ventral to the 4th ventricle cerebellum b. Alar plate is displaced lateral to basal plate 4 Medulla Developing Neural Tube 2. Cranial nerve nuclei form discontinuous columns Rostral 12 SE Page 48 Notes 3. Some cranial nerve nuclei migrate from their primitive embryonic positions (e.g., nuclei of V and VII) Facial N. Factors responsible for the complex internal organization of the brainstem: 4) Special senses develop in association with the brain stem. Nuclei of special senses 5) Development of the cerebellum and its connections Cerebellum II. Cranial Nerve Nuclei: Nucleus = column of neuron cell bodies. Efferent nuclei are composed of cell bodies of alpha or gamma motor neurons (SE) or preganglionic parasympathetic neurons (VE). III. Motor Efferent Nuclei (Basal Plate Derivatives): 1. SE (Somatic Efferent) Nuclei: SE neurons form two longitudinally oriented but discontinuous columns of cell bodies in the brain stem. Neurons that comprise these columns are responsible for innervating all of the skeletal musculature of the head. -

What Is Optic Nerve Hypoplasia?
What is the optic nerve? The optic nerve is a collection of more than a million nerve fibers that transmit visual signals from the eye to the brain [See Figure 1]. The optic nerve develops during the first trimester of intrauterine life. Fig. 1: Normal optic nerve. What is optic nerve hypoplasia? Optic nerve hypoplasia (ONH) is a congenital condition in which the optic nerve is underdeveloped (small) [See figure 2]. Fig. 2: Optic nerve hypoplasia. How is optic nerve hypoplasia diagnosed? The diagnosis of ONH is typically made by the appearance of small/pale optic nerve during a dilated eye exam. It is difficult to predict visual acuity potential based on the optic nerve appearance. What causes optic nerve hypoplasia? Most cases of ONH have no clearly identifiable cause. There are no known racial or socioeconomic factors in the development of ONH, nor is there a known association with exposure to pesticides. ONH has been associated with maternal ingestion of phenytoin, quinine, and LSD, as well as with fetal alcohol syndrome. What visual problems are associated with optic nerve hypoplasia? Vision impairment from ONH ranges from mild to severe and may affect one or both eyes. Nystagmus (shaking of the eyes) may be seen with both unilateral and bilateral cases. The incidence of strabismus is increased with ONH. Is optic nerve hypoplasia associated with non-visual problems? Optic nerve hypoplasia can be associated with central nervous system (CNS) malformations which put the patient at risk for other problems, including seizure disorder and developmental delay. Hormone deficiencies occur in most children, regardless of associated midline brain abnormalities or pituitary gland abnormalities on MRI.