B.Sc Course Matrix I Semester B.Sc Marks Part Paper Title Hours IA Exam Total Credits
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

M.Com (Master of Commerce) Affiliated Colleges Lists
Note:- It is hereby informed to all the Students /Parents/Guardians are to see the list of colleges display who are seeking admission for M.Com for various courses of BUB. Decide to choose the colleges to get admission. After admission, Under no circumstances students are allowed to change the college M.Com (Master of Commerce) Affiliated Colleges Lists Sl.No College Name Phone Number Address Sri Gokula College of Arts, Science & Mgt.Studies, 1 9449619597 Gokul Nagar, Bengaluru-Chennai Bypass Road , Kolar-563101 Kolar 2 Smt.Danamma Chennabasavaiah Mahila Samaja 8152220400 Doom light circle Kolar -563101 Ayyappanagar Circle, Devasandra Main Road Virgonagar post 3 SEA College of Arts,Science & Commerce, KR Puram 25613741 / 65470229 / 25613742 k.R Puram Bangalore-49 4 Padmashree Institute of Mgt. Science, Kengeri 9880430827/28485204/28485205 No 149, Kommaghatta sulikere (post) Kengeri Bangalore-60 5 Sri Konagadiyappa College , Doddaballapura 9448076253 / 27623759 Doddaballapura -561203 9880941320 / 22955371 / 6 Seshadripuram First Grade College,College Yelahanka No 26,Yelahanka New Town Bengaluru -64 22955369 7 Hasnath College (Co-education) Kalyana nagar Hennuru road Bangalore -43 Al-Ameen College of Arts,Science & Commerce, 9880740314 / 22222402 / 8 near Lalbagh Main Gate Hosur Road Bangalore-27 Lalbagh 22235626 9 Dr. Ambedkar Insttute of Mgt. Studies, Indiranagar 9008144500 / 25274994 H.A.L 2nd Stage Indiranagar , Bangalore-8 10 Acharya Institute Management & Science, Peenya 9945421819 1st Cross 1st stage Peenya bangalore 58 11 Acharya -

In the High Court of Karnataka at Bengaluru
IN THE HIGH COURT OF KARNATAKA AT BENGALURU Dated this the 24th day of July, 2015 Present THE HON’BLE MR JUSTICE VINEET SARAN & THE HON’BLE MR JUSTICE RAGHVENDRA S CHAUHAN Review Petition 287 / 2015 in WP (HC) 234 / 2014 Between 1 Smt Katamma, 65 yrs W/o Muniraju @ Haddugadu R/a # 1, in Sy.No.135 & 136 of Binnamangala Village Old Madras Road, Indiranagar Bangalore 2 Sri M B Ramu, 64 yrs S/o late M B Muniyappa R/a # 50/1, Upper Pipe Line Seshadripuram, Bangalore 20 Petitioners (By Sri Bryen for Sri M Manjunath, Adv.) And 1 Commissioner of Police Infantry Road, Bangalore 2 Station House Officer Indiranagar Police Station Bangalore 38 3 Sri N Sampath Kumar, 53 yrs S/o late V Narasimhulu R/a # 48, Near Ganesh Temple 2 Hutting Colony, Indiranagar I Stage Bangalore 38 4 Sri D Ramaraju, 55 yrs S/o late Doddamarappa R/a # 19/3, New Binnamangala Old Madras Road, Bangalore 5 Sri M Ravikumar, 43 yrs S/o late M Muniraju R/a # 30, 3 rd Cross, V R Road Ramamurthy Nagar, Bangalore 6 Sri M Chandrashekar, 40 yrs S/o late M Muniraju R/a # 8/9, Laxmi Narasimha Nilaya Laxmi Tent Road, 8 th Cross Ramamurthy Nagar, Bangalore 7 Sri M Muralidhara, 39 yrs S/o late M Muniraju R/a # 16, Near Mother Theresa School Behind Aiyappa Nagar Layout Maragundana Halli Main Road (Anandapura), Varanasi Jinka Thimmana Halli Village Bangalore 8 Sri Umashankar S/o Doddamarappa 52 yrs, R/a # 2, Subramanya Temple Street Old Byapanahalli, Bangalore 33 9 Sri R Shekar @ Sheiki, 54 yrs S/o late Rajappa, R/a # 495, TBW Near Park, 15 th Cross, Indiranagar II Stage, Bangalore 38 10 Smart Investment & Holdings # 212, Copper Arch, 83, Infantry Road Bangalore - by Partners Mr Irshad Ahmed & Mr Keshav R 3 11 Shell India Markets Pvt Ltd Br. -

1 in the High Court of Karnataka at Bangalore
1 IN THE HIGH COURT OF KARNATAKA AT BANGALORE DATED THIS THE 20 TH DAY OF JULY 2012 PRESENT THE HON’BLE MR.VIKRAMAJIT SEN, CHIEF JUSTICE AND THE HON’BLE MR.JUSTICE S.N.SATYANARAYANA R.P.No.66/2012 IN W A No. 1431/2008 & WA Nos.1265-66/2011 C/w. R P No. 67/2012 IN W A No.1424/2008 & WA Nos.1142-64/2011 R P No. 68/2012 IN W A No. 1423/2008 & WA Nos.1267-74/2011 R.P.No.66/2012 IN W A No. 1431/2008 & WA Nos.1265-66/2011 BETWEEN: 1. M/S SURABHI SEVA SANGHA REGD., NO.187, 22 ND CROSS, 6 TH BLOCK, JAYANAGAR, BANGALORE-82 BY ITS SECRETARY SHRI CHANDRASHEKARAIAH S/O BATTAIAH, AGED ABOUT 63 YEARS, R/O NO.228/E, 10 TH CROSS,9 TH MAIN, K.S.R.T.C LAYOUT, J P NAGAR, 2ND PHASE, BANGALORE – 560 078. 2. SHRI S. SUBBARAO S/O LATE KRISHNAMURTHY, AGED ABOUT 75 YEARS, SENIOR CITIZEN R/O NO.187, 22 ND CROSS, 6TH BLOCK, JAYANAGARA, BANGALORE-560 082. 3. SHRI B C MUDDUMADAPPA S/O LATE CHIKKAIAH, AGED ABOUT 87 YEARS R/O 196, 6TH BLOCK, 23 RD CROSS, JAYANAGAR, BANGALORE-560 082. ... PETITIONERS (BY SRI M R NAIK, SR. COUNSEL A/W SRI M R RAJAGOPAL, ADV.) 2 AND: 1. THE STATE OF KARNATAKA BY ITS SECRETARY, DEPARTMENT OF HOUSING AND URBAN DEVT., VIKASA SOUDHA, BANGALORE. 2. THE COMMISSIONER BANGALORE DEVELOPMENT AUTHORITY, KUMARA PARK WEST, BANGALORE-560 020. 3. THE DEPUTY COMMISSIONER LAND ACQUISITION, BANGALORE DEVELOPMENT AUTHORITY, KUMARA PARK WEST, BANGALORE-560 020. -
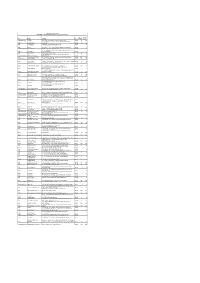
FOLIO NAME 1 ADDRESS PIN NO. of SHARES DIV AMT (In Rs.) IN30113526141278 a a SINDHU NO 5 PRABHATH,3RD MAIN VYALIKAVAL,BANGAL
KENNAMETAL INDIA LIMITED UNCLAIMED / UNPAID DIVIDEND DATA 2014-15 (I) AS ON 30-04-2018 NO. OF DIV AMT FOLIO NAME_1 ADDRESS PIN SHARES (in Rs.) IN30113526141278 A A SINDHU NO 5 PRABHATH,3RD MAIN VYALIKAVAL,BANGALORE 560003 100 200 97 3RD MAIN 2ND CROSS,MICO LAYOUT, MAHALAKSHMIPURAM, A0675 A B MENDONCE BANGALORE 560086 80 160 A0663 A C POOVANNA F-301 PURVA PAVILION,HEBBAL,BANGALORE 560024 5 10 A0666 A C POOVANNA F 301 PURVA PAVILION,HEBBAL,BANGALORE 560024 1 2 A0662 A GOPAL KENNAMETAL INDIA LTD,8/9TH MILE,TUMKUR ROAD,BANGALORE 560073 1 2 IN30192630446648 A S ASHOK KUMAR NO H-93,TANK ROAD,,DODDABALLAPUR 561203 5 10 200269 KENNAMETAL INDIA LTD,8/9TH MILE TUMKUR ROAD,(R & D EPG A0673 A S NAGARAJ DEPT),BANGALORE 560073 1 2 CK237 ABHAI KUMAR 3/17 JAWAHAR NAGAR,JAIPUR 0 200 400 WARD NO 4,ADINATH AGENCIES,SHIVAJI NAGAR, KHAMGAON 1302310000042420 ABHAY VIJAY ZAMBAD ROAD,NANDURA 443404 3 6 CA007 AHMED MOHAMED AFINIA JANTA COLONY B 3/41,SINGH NIWAS,JOGESHWARI EAST,MUMBAI 400060 2880 5760 1203470000002215 AMEET GANGULY I - 1647,C. R. PARK,NEW DELHI 110019 100 200 A0413 AMITA JINDAL C/O DR. PAWAN JINDAL,137, URBAN ESTATE,SECTOR 7,AMBALA CITY (HR) 134002 20 40 A0653 ANAHITA A KOHLI 1/20 KUMAR CITY,KALYANI NAGAR,,PUNE 411006 1000 2000 A0360 ANAND NARAYAN TANDON 41A, GREENVIEW APARTMENT,SECTOR 15A, NOIDA 201301 200 400 NO 97 6TH CROSS,32ND MAIN ITI LAYOUT,J P NAGAR IST A0672 ANAND SINGH C J PHASE,BANGALORE 560078 1 2 9/17, CHANDRANAGAR HOUSING,SOCIETY, POONA-SATARA ROAD,,OPP. -

Portrayal of Family Ties in Ancient Sanskrit Plays: a Study
Volume II, Issue VIII, December 2014 - ISSN 2321-7065 Portrayal of Family Ties in Ancient Sanskrit Plays: A Study Dr. C. S. Srinivas Assistant Professor of English Mahatma Gandhi Institute of Technology Hyderabad India Abstract The journey of the Indian dramatic art begins with classical Sanskrit drama. The works of the ancient dramatists Bhasa, Kalidasa, Bhavabhuti and others are the products of a vigorous creative energy as well as sustained technical excellence. Ancient Sanskrit dramatists addressed several issues in their plays relating to individual, family and society. All of them shared a common interest— familial and social stability for the collective good. Thus, family and society became their most favoured sites for weaving plots for their plays. Ancient Sanskrit dramatists with their constructive idealism always portrayed harmonious filial relationships in their plays by persistently picking stories from the two great epics Ramayana and Mahabharata and puranas. The paper examines a few well-known ancient Sanskrit plays and focuses on ancient Indian family life and also those essential human values which were thought necessary and instrumental in fostering harmonious filial relationships. Keywords: Family, Filial, Harmonious, Mahabharata, Ramayana, Sanskrit drama. _______________________________________________________________________ http://www.ijellh.com 224 Volume II, Issue VIII, December 2014 - ISSN 2321-7065 The ideals of fatherhood and motherhood are cherished in Indian society since the dawn of human civilization. In Indian culture, the terms ‘father’ and ‘mother’ do not have a limited sense. ‘Father’ does not only mean the ‘male parent’ or the man who is the cause of one’s birth. In a broader sense, ‘father’ means any ‘elderly venerable man’. -

Karnataka 1. Dr. K.G.Balakrishnan E 135, Sobha Hibiscus, Sarjapur
Karnataka 1. Dr. K.G.Balakrishnan 2. Dr. Vijayalakshmi I. Balekundra E 135, Sobha Hibiscus, Aditi, 44A, V Main Road Sarjapur Outer Ring Road, Vijayanagar II Stage Bellandur, Bangalore-560103 Hampinagar Bangalore-560040 3. Dr.Asit Kumar Chakraborty 4. Dr. A. Chandramuki "Bikalpa", 557, 526, 11th Main, 4th Block, 8th Main, Jayanagar, Koramangala, 5th Block, Bangalore-560034. Bangalore-560041. 5. Dr. S Prabha Chandra 6. Dr. George Cherian Professor, House No. 828, Department of Psychiatry, 13th Main Road, National Institute of Mental Health 3 Block, Koramangala, and Neuro Sciences, Bangalore – 560034 Bangalore-560029 7. Dr. Bikramjit Basu 8. Dr. B.N. Gangadhar Material Research Center Professor Indian Institute of Sciences Deptt. of Psychiatry Bangalore-560012 National Instt. of Mental Health & Karnataka Neurosciences, P.O. Box 2900, Hosur Road, Bangalore-560028 9. Dr.K.S. Gopinath 10. Dr. Gomathy Gopinath Director, Flat No. 001 Consultant Surgical Oncologist, Kanchanjunga Apptts. Bangalore Institute of Oncology, 122/2, Nagavarapalya 44-45/2, 2nd Cross, C.V. Raman Nagar R.M.R.R. Exten., Bangalore – 560093 Bangalore-560027. 11. Dr.P.G.Gopinathan Nair 12. Dr. Chhitar Mal Gupta, FAMS Flat No. 001 Appt. No. D-508, Raheja Residency, Ground Floor 3rd Block, Koramangala, Kanchanjunga Apptts. Bangaluru-560034 122/2, Nagavarapalya C.V. Raman Nagar Bangalore – 560093 13. Dr. Gopalkrishna Gururaj 14. Dr. C.V. Harinarayan No. 74, Unnathi, Shree, No 260, 4th Main Road Mountain Road, (Near Ayyappa Temple) 1st Block East, Jayanagar, Vijaya Bank Colony Bangalore-560011 (Behind IIM-B), Bangalore-560076 15. Dr.B.M.Hegde 16. Dr. Usha Kini Manju Nath, 524, 20th Main Pai Hills, Bijai 4th T Block, Jayanagar Mangalore - 575004 Bangalore-560041 17. -

Buddhacarita
CLAY SANSKRIT LIBRARY Life of the Buddka by AsHvaghosHa NEW YORK UNIVERSITY PRESS & JJC EOUNDATION THE CLAY SANSKRIT LIBRARY FOUNDED BY JOHN & JENNIFER CLAY GENERAL EDITORS RICHARD GOMBRICH SHELDON POLLOCK EDITED BY ISABELLE ONIANS SOMADEVA VASUDEVA WWW.CLAYSANSBCRITLIBRARY.COM WWW.NYUPRESS.ORG Copyright © 2008 by the CSL. All rights reserved. First Edition 2008. The Clay Sanskrit Library is co-published by New York University Press and the JJC Foundation. Further information about this volume and the rest of the Clay Sanskrit Library is available at the end of this book and on the following websites: www.ciaysanskridibrary.com www.nyupress.org ISBN-13: 978-0-8147-6216-5 (cloth : alk. paper) ISBN-10: 0-8147-6216-6 (cloth : alk. paper) Artwork by Robert Beer. Typeset in Adobe Garamond at 10.2$ : 12.3+pt. XML-development by Stuart Brown. Editorial input from Linda Covill, Tomoyuki Kono, Eszter Somogyi & Péter Szântà. Printed in Great Britain by S t Edmundsbury Press Ltd, Bury St Edmunds, Suffolk, on acidffee paper. Bound by Hunter & Foulis, Edinburgh, Scotland. LIFE OF THE BUDDHA BY ASVAGHOSA TRANSLATED BY PATRICK OLIVELLE NEW YORK UNIVERSITY PRESS JJC FOUNDATION 2008 Library of Congress Cataloging-in-Publication Data Asvaghosa [Buddhacarita. English & Sanskrit] Life of the Buddha / by Asvaghosa ; translated by Patrick Olivelle.— ist ed. p. cm. - (The Clay Sanskrit library) Poem. In English and Sanskrit (romanized) on facing pages. Includes bibliographical references and index. ISBN-13: 978-0-8147-6216-5 (cloth : alk. paper) ISBN-10: 0-8147-6216-6 (cloth : alk. paper) 1. Gautama Buddha-Poetry. I. Olivelle, Patrick. II. -

2017-18 Guidance Value for the Immovable Properties Coming Under the Jurisdiction of Basavanagudi Sub-Register Office
2017-18 Guidance value for the Immovable Properties coming under the jurisdiction of Basavanagudi Sub-Register Office. Residential Sites coming under Apartments/Flats the Jurisdiction of Local Constructed on Residential Sites approved Organization SI NO Hobli/Village/Ward/Road/Area Name by competent Authority/Local Organization (Rupees per Square Meter For Super (Rupees in Lakhs per Acre) Built up Area) 1 2 3 4 A SRINAGARA 1 Srinagara Main Road 49700 2 Srinagara Cross Road 46200 3 Anantha Murthy Layout 37900 4 Srinagara Pipe Line 37900 5 Brindavana Nagar 37900 6 Dasarahalli 37900 7 Kalidasa Layout 37900 8 Srinagar 13th Main Road 60000 9 10th Main Road 60000 10 12th Main Road 60000 11 14th Main Road 60000 12 Kalappa Block 55000 13 8th Main Road 58000 14 9th Main Road 60000 15 Other Areas 37900 B BASAVANAGUDI 16 Gandhi Bazaar 108700 17 D.V.G. Road 90400 18 Sowmya Springs Apartment 78100 Ananda Apartment 5th Main K.G Nagar, 19 90000 Basavanagudi Marques Apartments Siddh Shekha 20 90000 Developers No 98 Basavanagudi 21 Brindavan Apartment 78100 22 Shreshta Bhoomi Apartment 79500 23 Patalamma Road 92600 24 Puttanna Road 65100 25 Krishna Residency 54400 26 Lakshmi Residency 54400 27 Dewan Madhav Rao Road 86700 28 Mohammedan Block 54500 29 Khaji Street 54500 30 Ranoji Rao Road 54500 31 Vani Vilas Road 86700 32 Vani Vilas Cross Road 67800 33 Bull Temple Road 90400 34 Parshwanath Apartment 84300 35 Platinum Anand Apartment 84300 36 Brindavan Mansion Apartment 72700 37 Pranag Arch 72700 38 Bull Temple Cross Road 76400 39 Surveyor Street 64000 40 Govindappa Road 64000 41 Symbiosis Royal Apartment 57500 42 K.R. -

Diversity of Animals 355 15 | DIVERSITY of ANIMALS
Concepts of Biology Chapter 15 | Diversity of Animals 355 15 | DIVERSITY OF ANIMALS Figure 15.1 The leaf chameleon (Brookesia micra) was discovered in northern Madagascar in 2012. At just over one inch long, it is the smallest known chameleon. (credit: modification of work by Frank Glaw, et al., PLOS) Chapter Outline 15.1: Features of the Animal Kingdom 15.2: Sponges and Cnidarians 15.3: Flatworms, Nematodes, and Arthropods 15.4: Mollusks and Annelids 15.5: Echinoderms and Chordates 15.6: Vertebrates Introduction While we can easily identify dogs, lizards, fish, spiders, and worms as animals, other animals, such as corals and sponges, might be easily mistaken as plants or some other form of life. Yet scientists have recognized a set of common characteristics shared by all animals, including sponges, jellyfish, sea urchins, and humans. The kingdom Animalia is a group of multicellular Eukarya. Animal evolution began in the ocean over 600 million years ago, with tiny creatures that probably do not resemble any living organism today. Since then, animals have evolved into a highly diverse kingdom. Although over one million currently living species of animals have been identified, scientists are [1] continually discovering more species. The number of described living animal species is estimated to be about 1.4 million, and there may be as many as 6.8 million. Understanding and classifying the variety of living species helps us to better understand how to conserve and benefit from this diversity. The animal classification system characterizes animals based on their anatomy, features of embryological development, and genetic makeup. -

Deep-Sea Video Technology Tracks a Monoplacophoran to the End of Its Trail (Mollusca, Tryblidia)
Deep-sea video technology tracks a monoplacophoran to the end of its trail (Mollusca, Tryblidia) Sigwart, J. D., Wicksten, M. K., Jackson, M. G., & Herrera, S. (2018). Deep-sea video technology tracks a monoplacophoran to the end of its trail (Mollusca, Tryblidia). Marine Biodiversity, 1-8. https://doi.org/10.1007/s12526-018-0860-2 Published in: Marine Biodiversity Document Version: Publisher's PDF, also known as Version of record Queen's University Belfast - Research Portal: Link to publication record in Queen's University Belfast Research Portal Publisher rights Copyright 2018 the authors. This is an open access article published under a Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the author and source are cited. General rights Copyright for the publications made accessible via the Queen's University Belfast Research Portal is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The Research Portal is Queen's institutional repository that provides access to Queen's research output. Every effort has been made to ensure that content in the Research Portal does not infringe any person's rights, or applicable UK laws. If you discover content in the Research Portal that you believe breaches copyright or violates any law, please contact [email protected]. Download date:06. Oct. 2021 Deep-sea video technology tracks a monoplacophoran to the end of its trail (Mollusca, Tryblidia) Sigwart, J. -

URUBHANGAM (BREAKING of THIGHS) – a TRAGEDY in INDIAN TRADITION Bhagvanbhai H.Chaudhari, Ph. D. Assoc. Professor, Dept. Of
SRJIS/BIMONTHLY/ DR. BHAGVANBHAI H. CHAUDHARI (5683-5688) URUBHANGAM (BREAKING OF THIGHS) – A TRAGEDY IN INDIAN TRADITION Bhagvanbhai H.Chaudhari, Ph. D. Assoc. Professor, Dept. of English, The KNSBL Arts and Commerce College, Kheralu Gujarat (India) Scholarly Research Journal's is licensed Based on a work at www.srjis.com By and large a play is considered an „imitation of folk-attitude‟ wherein the outcome of human activity may either be happy or unhappy. Since the time of ancient Greek literature in the West, the drama has been categorized as comedy and tragedy. But Bharat Muni in his Natyashastra projected it to be: (एतद्रसेषु भावेषु सववकमवक्रियास्वथ । सवोऩदेशजननं ना絍यं ऱोके भववष्यतत ॥ ) दԃु खातावनां श्रमातावनां शोकातावनां तऩस्स्वनाम ्। ववश्रास्ततजननं काऱे ना絍यमेतद्भववष्यतत ॥ ११४॥ धर्म्यं यशस्यमायुष्यं हहतं बुविवववधनव म ् । ऱोकोऩदेशजननं ना絍यमेतद्भववष्यतत ॥ ११५॥ ( १) i.e. It will [also] give relief to unlucky persons who are afflicted with sorrow and grief or [over]-work, and will be conducive to observance of duty(dharma) as well as to fame, long life, intellect and general good, and will educate people. (Ghosh 15) Further, he explains the concept and the significance of drama as ईश्वराणां ववऱासश्च स्थैयं दԃु खाहदवतस्य च । अथोऩजीववनामथो धतृ त셁饍वेगचते साम ्॥ १११॥ MAY-JUNE 2017, VOL- 4/31 www.srjis.com Page 5683 SRJIS/BIMONTHLY/ DR. BHAGVANBHAI H. CHAUDHARI (5683-5688) नानाभावोऩसर्म्ऩतनं नानावस्थाततरा配मकम ्। ऱोकव配ृ तानुकरणं ना絍यमेततमया कृ तम ्॥ ११२॥ i.e. -

Mahabharata in Visual and Performing Arts: Texts, Contexts and Images
Seminar on Text and Variation of the Mahabarata Session III: Mahabharata in visual and performing Arts: Texts, Contexts and Images MAHABHARATA AS REPRESENTED IN THE CLASSICAL AND CONTEMPORARY THEATRE OF KERALA Dr. K.G. Paulose Three ancient texts – Ramayana, Mahabharata and Bhagavata has moulded the mindset of Indians for centuries. Ramayana is the model for intra-domestic affairs, Mahabharata for interaction with society and Bhagavata for spiritual purity. Mahabharata, unlike the other two, is not permitted to be used for regular chanting for fear of creating quarrel in the household.1 Mahabharata presents man as he is where as the other two depicts a sophisticated and idealized levels of living. It is precisely because of this that theatre likes Mahabharata more than anything else. The influence of Mahabharata on theatre is tremendous. Bhasa and Kalidasa Mahabharata has been a veritable source for Sanskrit dramatists to develop their themes. Bhasa and Kalidasa were the earliest playwrights who were inspired by the great epic. Of the two, Bhasa revolted, often amending Vyasa by suitable substitutes and filling his silence with own interpretations. Kalidasa, on the other hand, often compromised to the epic narrative. Bhasa was sympathetic towards the characters who were marginalized, neglected and condemned - Karna, Duryodhana, Gatotkacha, etc.. He made them heroes. Krishna, Dharmaputra or even Arjuna became pale in their presence. In Pancharatra Bhasa goes to the extent of suggesting an alternative to Vyasa.2 Vyasa tells us that there is no alternative to bloodshed to solve the Kuru-Pandhava feud. It is an indirect approval for warfare. But Bhasa amends and corrects that there are alternatives, the way of negotiations, peaceful settlements.