Latestvmlist.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Product List March 2019 - Page 1 of 53
Wessex has been sourcing and supplying active substances to medicine manufacturers since its incorporation in 1994. We supply from known, trusted partners working to full cGMP and with full regulatory support. Please contact us for details of the following products. Product CAS No. ( R)-2-Methyl-CBS-oxazaborolidine 112022-83-0 (-) (1R) Menthyl Chloroformate 14602-86-9 (+)-Sotalol Hydrochloride 959-24-0 (2R)-2-[(4-Ethyl-2, 3-dioxopiperazinyl) carbonylamino]-2-phenylacetic 63422-71-9 acid (2R)-2-[(4-Ethyl-2-3-dioxopiperazinyl) carbonylamino]-2-(4- 62893-24-7 hydroxyphenyl) acetic acid (r)-(+)-α-Lipoic Acid 1200-22-2 (S)-1-(2-Chloroacetyl) pyrrolidine-2-carbonitrile 207557-35-5 1,1'-Carbonyl diimidazole 530-62-1 1,3-Cyclohexanedione 504-02-9 1-[2-amino-1-(4-methoxyphenyl) ethyl] cyclohexanol acetate 839705-03-2 1-[2-Amino-1-(4-methoxyphenyl) ethyl] cyclohexanol Hydrochloride 130198-05-9 1-[Cyano-(4-methoxyphenyl) methyl] cyclohexanol 93413-76-4 1-Chloroethyl-4-nitrophenyl carbonate 101623-69-2 2-(2-Aminothiazol-4-yl) acetic acid Hydrochloride 66659-20-9 2-(4-Nitrophenyl)ethanamine Hydrochloride 29968-78-3 2,4 Dichlorobenzyl Alcohol (2,4 DCBA) 1777-82-8 2,6-Dichlorophenol 87-65-0 2.6 Diamino Pyridine 136-40-3 2-Aminoheptane Sulfate 6411-75-2 2-Ethylhexanoyl Chloride 760-67-8 2-Ethylhexyl Chloroformate 24468-13-1 2-Isopropyl-4-(N-methylaminomethyl) thiazole Hydrochloride 908591-25-3 4,4,4-Trifluoro-1-(4-methylphenyl)-1,3-butane dione 720-94-5 4,5,6,7-Tetrahydrothieno[3,2,c] pyridine Hydrochloride 28783-41-7 4-Chloro-N-methyl-piperidine 5570-77-4 -

B Commission Regulation (Eu)
02010R0037 — EN — 29.09.2018 — 035.001 — 1 This text is meant purely as a documentation tool and has no legal effect. The Union's institutions do not assume any liability for its contents. The authentic versions of the relevant acts, including their preambles, are those published in the Official Journal of the European Union and available in EUR-Lex. Those official texts are directly accessible through the links embedded in this document ►B COMMISSION REGULATION (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin (Text with EEA relevance) (OJ L 15, 20.1.2010, p. 1) Amended by: Official Journal No page date ►M1 Commission Regulation (EU) No 758/2010 of 24 August 2010 L 223 37 25.8.2010 ►M2 Commission Regulation (EU) No 759/2010 of 24 August 2010 L 223 39 25.8.2010 ►M3 Commission Regulation (EU) No 761/2010 of 25 August 2010 L 224 1 26.8.2010 ►M4 Commission Regulation (EU) No 890/2010 of 8 October 2010 L 266 1 9.10.2010 ►M5 Commission Regulation (EU) No 914/2010 of 12 October 2010 L 269 5 13.10.2010 ►M6 Commission Regulation (EU) No 362/2011 of 13 April 2011 L 100 26 14.4.2011 ►M7 Commission Regulation (EU) No 363/2011 of 13 April 2011 L 100 28 14.4.2011 ►M8 Commission Implementing Regulation (EU) No 84/2012 of 1 L 30 1 2.2.2012 February 2012 ►M9 Commission Implementing Regulation (EU) No 85/2012 of 1 L 30 4 2.2.2012 February 2012 ►M10 Commission Implementing Regulation (EU) No 86/2012 of 1 L 30 6 2.2.2012 February 2012 ►M11 Commission -

Report Name:Ukraine's Mrls for Veterinary Drugs
Voluntary Report – Voluntary - Public Distribution Date: November 05,2020 Report Number: UP2020-0051 Report Name: Ukraine's MRLs for Veterinary Drugs Country: Ukraine Post: Kyiv Report Category: FAIRS Subject Report Prepared By: Oleksandr Tarassevych Approved By: Robin Gray Report Highlights: Ukraine adopted several maximum residue levels (MRLs) for veterinary drugs, coccidiostats and histomonostats in food products of animal origin. Ukraine also adopted a list of drugs residues that are not allowed in food products. THIS REPORT CONTAINS ASSESSMENTS OF COMMODITY AND TRADE ISSUES MADE BY USDA STAFF AND NOT NECESSARILY STATEMENTS OF OFFICIAL U.S. GOVERNMENT POLICY The Office of Agricultural Affairs of USDA/Foreign Agricultural Service in Kyiv, Ukraine prepared this report for U.S. exporters of domestic food and agricultural products. While every possible care was taken in the preparation of this report, information provided may not be completely accurate either because policies have changed since the time this report was written, or because clear and consistent information about these policies was not available. It is highly recommended U.S. exporters verify the full set of import requirements with their foreign customers, who are normally best equipped to research such matters with local authorities, before any goods are shipped. This FAIRS Subject Report accompanies other reports on Maximum, Residue Limits established by Ukraine in 2020. Related reports could be found under the following links: 1.) Ukraine's MRLs for Microbiological Contaminants_Kyiv_Ukraine_04-27-2020 2.) Ukraine's MRLs for Certain Contaminants_Kyiv_Ukraine_03-06-2020 Food Products of animal origin and/or ingredients of animal origin are not permitted in the Ukrainian market if they contain certain veterinary drugs residues in excess of the maximum residue levels established in Tables 1 and 2. -

Download Drug Labels List
Syringe Labelling System Price Per Label Description/Drug Name Item No. Quanitiy Per Pack Pack Abciximab 99801 2 x 500 roll's £6.30 Abidec 100602 2 x 500 roll's £6.30 Acepromazine 99802 2 x 500 roll's £6.30 Acetazolamide 99803 2 x 500 roll's £6.30 Acetylcholine 99804 2 x 500 roll's £6.30 Acetylcysteine 99805 2 x 500 roll's £6.30 Acetylsalicylic Acid 99806 2 x 500 roll's £6.30 Aciclovir 99807 2 x 500 roll's £6.30 ACP/Buprenorphine 100208 2 x 500 roll's £6.30 Actrapid Insulin 99808 2 x 500 roll's £6.30 Adenosine 99809 2 x 500 roll's £6.30 Adrenaline (Top Half Black, Bottom Violet, Violet Text) 99810 2 x 500 roll's £6.30 Adrenaline/Epinephrine 99811 2 x 500 roll's £6.30 Albumin Solution 99812 2 x 500 roll's £6.30 Alchol 99813 2 x 500 roll's £6.30 Alemtuzmab 99814 2 x 500 roll's £6.30 ALERT 100243 2 x 500 roll's £6.30 Alfaxalone 99815 2 x 500 roll's £6.30 Alfentanil 99816 2 x 500 roll's £6.30 Alfentanil 99817 2 x 500 roll's £6.30 Alteplase 99818 2 x 500 roll's £6.30 Amikacin 99819 2 x 500 roll's £6.30 Aminophylline 99820 2 x 500 roll's £6.30 Amiodarone 100194 2 x 500 roll's £6.30 Amoxicillin 100195 2 x 500 roll's £6.30 Amphotericin 99821 2 x 500 roll's £6.30 Ampicillin 99822 2 x 500 roll's £6.30 Antibiotic 99823 2 x 500 roll's £6.30 Anticoagulant 99824 2 x 500 roll's £6.30 Antifungal 100228 2 x 500 roll's £6.30 Antiseptic 99825 2 x 500 roll's £6.30 Aprotinin 99826 2 x 500 roll's £6.30 Aqueous Iodine 99827 2 x 500 roll's £6.30 Arterial 100259 2 x 500 roll's £6.30 Arterial ( Line Label - White with Red Writing) 100176 2 x 500 roll's £6.30 Arterial -

Download PDF (Inglês)
Braz. J. vet. Res. anim. Sci., São Paulo, v. 38, n. 3, p. 142-145, 2001. CORRESPONDENCE TO: Lecirelin and Buserelin (Gonadotrophin Pietro Sampaio Baruselli Departamento de Reprodução Animal da Faculdade de Medicina releasing hormone agonists) are equally effective Veterinária e Zootecnia da USP Av. Prof. Dr. Orlando Marques de for fixed time insemination in buffalo Paiva, 87 Cidade Universitária Armando de Salles Oliveira 05508-000 – São Paulo – SP A Lecirelina apresenta eficiência similar à da Buserelina e-mail: [email protected] 1- Departamento de Reprodução (agonistas do hormônio liberador de Gonadotrofinas) Animal da Faculdade de Medicina Veterinária e Zootecnia da USP – SP para inseminação artificial em tempo fixo em bubalinos 2- Médico Veterinário Autônomo Pietro Sampaio BARUSELLI1; Renato AMARAL2; Francisco Bonomi BARUFI1; Renato VALENTIM1; Marcio de Oliveira MARQUES1 SUMMARY Buffalo has peculiar reproductive patterns, which make artificial insemination programs a hard and expensive task. Artificial insemination in fixed time is advantaged because females show low incidence of homosexual behaviour and strong dominance relationships, which leads to a poor accuracy in estrus detection. The aim of this experiment was to compare the efficiency of two different GnRH agonists in the GnRH/PGF2α/GnRH protocol (Buserelin vs Lecirelin). Two hundred and seventy buffaloes with 45 to 60 days postpartum were synchronized and fixed-time inseminated. The animals were kept on pasture in two farms at São Paulo and Mato Grosso do Sul (Brazil). Cows in Group 1 (n = 132) received, intramuscularly, 20 µg of Buserelin at a random day of the estrous cycle and, seven days later, 15 mg of prostaglandin F2α. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

Fully Automated Dried Blood Spot Sample Preparation Enables the Detection of Lower Molecular Mass Peptide and Non-Peptide Doping Agents by Means of LC-HRMS
Analytical and Bioanalytical Chemistry (2020) 412:3765–3777 https://doi.org/10.1007/s00216-020-02634-4 RESEARCH PAPER Fully automated dried blood spot sample preparation enables the detection of lower molecular mass peptide and non-peptide doping agents by means of LC-HRMS Tobias Lange1 & Andreas Thomas1 & Katja Walpurgis1 & Mario Thevis1,2 Received: 10 December 2019 /Revised: 26 March 2020 /Accepted: 31 March 2020 # The Author(s) 2020 Abstract The added value of dried blood spot (DBS) samples complementing the information obtained from commonly routine doping control matrices is continuously increasing in sports drug testing. In this project, a robotic-assisted non-destructive hematocrit measurement from dried blood spots by near-infrared spectroscopy followed by a fully automated sample preparation including strong cation exchange solid-phase extraction and evaporation enabled the detection of 46 lower molecular mass (< 2 kDa) peptide and non-peptide drugs and drug candidates by means of LC-HRMS. The target analytes included, amongst others, agonists of the gonadotropin-releasing hormone receptor, the ghrelin receptor, the human growth hormone receptor, and the antidiuretic hormone receptor. Furthermore, several glycine derivatives of growth hormone–releasing peptides (GHRPs), argu- ably designed to undermine current anti-doping testing approaches, were implemented to the presented detection method. The initial testing assay was validated according to the World Anti-Doping Agency guidelines with estimated LODs between 0.5 and 20 ng/mL. As a proof of concept, authentic post-administration specimens containing GHRP-2 and GHRP-6 were successfully analyzed. Furthermore, DBS obtained from a sampling device operating with microneedles for blood collection from the upper arm were analyzed and the matrix was cross-validated for selected parameters. -
20201127-Brochure-Dalmarelin.Pdf
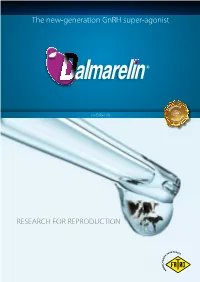
The new-generation GnRH super-agonist ® LECIRELIN RESEARCH FOR REPRODUCTION Lecirelin GnRH GnRH (Gonadotropin-Releasing A breakthrough Hormone), a decapeptide produced by neurons in the in Pharmaceutical Design basal hypothalamus, is the master hormone controlling and Engineering reproductive physiology. Gly NH2 It stimulates the synthesis Dalmarelin contains Lecirelin, a new-generation Pro and secretion of FSH GnRH (Follicle-Stimulating Hormone) GnRH super-agonist, which elicits a strong LH surge Arg and of LH (Luteinizing and a sustained FSH release from the Leu Hormone) from the anterior anterior pituitary gland. pituitary gland. LH and FSH Gly finally acts on the gonads to Tyr stimulate gametogenesis and Ser steroidogenesis and to regulate the estrous cycle. Trp R His ES EA pGlu R C H NEt Lecirelin D-tert Leu State-of-the-art research and technology have Lecirelin is a nonapeptide modeled after the natural GnRH decapeptide. The design of this GnRH agonist has been directed toward stabilization of the molecule and allowed to synthesize increasing its affinity for the GnRH receptor. a drug which shows Substitution of glycin at position 6 with D-tert-leucin has conferred greater stability against maximal potency, still enzymatic degradation and increased receptor binding affinity. Replacement of glycine by preserving a physiological ethylamide at position 10 has enhanced biological potency and conveyed further resistance to response proteolysis. ® Potent action, high efficacy For any of the clinical or management uses, the key biological response to evaluate efficacy of GnRH products is an adequate LH surge. AUCLH 20 Gonadorelin 100 µg 60 Lecirelin 25 µg 18 50 Lecirelin 50 µg LH (ng/ml) 16 Buserelin 10 µg 40 30 14 ng.h/ml 20 12 10 10 0 8 Gonadorelin Buserelin Lecirelin 25 Lecirelin 50 6 Time course of plasma LH Area under the LH concentrations (ng/mL) concentration curve 4 after administration of after administration gonadorelin, lecirelin and of gonadorelin, lecirelin 2 buserelin. -

Summary of Product Characteristics 1. Name Of
Revised: July 2020 AN: 00391/2020 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Chronogest CR, 20 mg controlled release vaginal sponge for sheep. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each polyester polyurethane sponge contains Active substance(s) Flugestone acetate, 20 mg. List of excipients Excipients qsp 1 sponge. For a full list of excipients, see section 6.1 3. PHARMACEUTICAL FORM Vaginal sponge. White cylindrical polyester polyurethane foam equipped with string. 4. CLINICAL PARTICULARS 4.1 Target species Sheep (ewes and ewe-lambs). 4.2 Indications for use In ewes and ewe lambs, in combination with PMSG (Pregnant Mare Serum Gonadotrophin) - Induction and synchronization of oestrus and ovulation (non cycling ewes during seasonal anoestrus and ewe lambs). - Synchronization of oestrus and ovulation (cycling ewes and ewe-lambs). 4.3 Contraindications Please refer to section 4.7 and section 4.8. 4.4 Special warnings None. Page 1 of 5 Revised: July 2020 AN: 00391/2020 4.5 Special precautions for use (i) Special precautions for use in animals - The repeated treatment with the product combined with PMSG may trigger the appearance of PMSG antibodies in some ewes. This in turn may affect the time of ovulation and result in reduced fertility when combined with fixed time artificial insemination at 55h following sponge removal. - The repeated use of sponges within one year has not been studied. - The use of a vaginal applicator designed for ewes or ewe lambs is recommended to correctly insert sponges and to avoid vaginal injuries. (ii) Special precautions to be taken by the person administering the medicinal product to animals - Direct contact with the skin should be avoided. -

RPB 2-2020.Pub
RESEARCH IN PIG BREEDING, 14, 2020 (2) WHICH HORMONES HELP TO OPTIMIZE PIGLET PRODUCTION? Wähner, M. Anhalt University of Applied sciences, Bernburg, Germany Abstract Sows are kept and piglet production nowadays generally according to the periodic group farrowing system with the use of artificial insemination. To this end, it is necessary to control the reproductive events in the sows. Their aim is to bring the sows in a group into heat almost at the same time so that they can be inseminated at the same time. This guarantees almost the same farrowing dates for the sows in a group. The most important measure in the sow group is at the end of the suckling period the date for the simultaneous weaning of the piglets from the sows. In order to support the synchronization of the reproductive processes in the sows in a group, the use of exogenous hormones in the respective cycle phases of the animals is recommended. In the article the hormones available for this (biotechnics) are presented with their effects. Reproductive hormones are differentiated according to their place of synthesis and their target organ. This includes the hypothalamus with the target organ pituitary gland (releasing hormone, GnRH), the anterior pituitary lobe with the gonads (gonadotropins, FSH, LH), the posterior pituitary lobe with the smooth muscles (oxytocin) and the sex steroids (prostaglandin) formed on the ovary (corpus luteum) call. As a tissue hormone, prostaglandin F2α is important with regard to its luteolytic effect. Finally, the environmentally relevant importance of hormone treatment in sows is discussed. Currently 10 to 15 percent of sows are treated with hormones. -

Low Doses of A2-Adrenoceptor Antagonists Augment Spinal Morphine Analgesia and Inhibit Development of Acute and Chronic Tolerance
British Journal of Pharmacology (2008) 155, 1264–1278 & 2008 Macmillan Publishers Limited All rights reserved 0007– 1188/08 $32.00 www.brjpharmacol.org RESEARCH PAPER Low doses of a2-adrenoceptor antagonists augment spinal morphine analgesia and inhibit development of acute and chronic tolerance B Milne, M Sutak, CM Cahill and K Jhamandas Department of Pharmacology and Toxicology, and Department of Anesthesiology, Queen’s University, Kingston, Ontario, Canada Background and purpose: Ultra-low doses of opioid receptor antagonists augment spinal morphine antinociception and block the induction of tolerance. Considering the evidence demonstrating functional and physical interactions between the opioid and a2-adrenoceptors, this study investigated whether ultra-low doses of a2-adrenoceptor antagonists also influence spinal morphine analgesia and tolerance. Experimental approach: Effects of low doses of the competitive a2-adrenoceptor antagonists—atipamezole (0.08, 0.8 ng), yohimbine (0.02, 2 ng), mirtazapine (0.02 ng) and idazoxan (0.08 ng) were investigated on intrathecal morphine analgesia, as well as acute and chronic morphine antinociceptive tolerance using the rat tail flick and paw pressure tests. Key results: At doses markedly lower than those producing a2-adrenoceptor blockade, atipamezole, yohimbine, mirtazapine and idazoxan, prolonged the antinociceptive effects of morphine. When co-administered with repeated acute spinal injections of morphine, all four agents blocked the induction of acute tolerance. Co-injection of atipamezole with morphine for 5 days inhibited the development of tolerance in a chronic treatment paradigm. Spinal administration of atipamezole also reversed established antinociceptive tolerance to morphine as indicated by the restoration of morphine antinociceptive potency. The effects of atipamezole on spinal morphine tolerance were not influenced by treatment with 6-hydroxydopamine. -

Supplementary Material Final Submitted
Supplementary material S1 Ciprofloxacin, Danofloxacin, Difloxacin, Enrofloxacin, Flumequine, Marbofloxacin, Nalidixic acid, Norfloxacin, Oxolinic acid, Sarafloxacin, Sulfaguanidine, Sulfanilamide, Sulfachlorpyridazine, Sulfadiazine, Sulfadimethoxine, Sulfadoxine, Sulfamerazine, Sulfamethazine, Sulfamethizole, Sulfamethoxypyridazine, Sulfamonomethoxine, Sulfapyridine, Sulfaquinoxaline, Sulfathiazole, Sulfisoxazole, Sulfamoxole, Trimethoprim, Sulfamethoxazole, Erythromycin, Josamycin, Lincomycin, Spiramycin, Tilmicosin, Tylosin A, Gamithromycin, Tildipirosin, Tulathromycin, Virginiamycin, Pirlimycin, Acetyltylosin-2-O, Tylvalosin, Valnemulin, Rifampicin, Dapsone, Chlortetracycline, Epichlortetracycline, Epitetracycline, Oxytetracycline, Epioxytetracycline, Doxycycline,Tetracycline, Phenoxymethylpenicillin, Benzylpenicillin, Amoxicillin, Ampicillin, Dicloxacillin, Cloxacillin, Nafcillin, Oxacillin, Cefalexin, Cefalonium, Cefapirin, Cefoperazone, Desacetylcefapirin, Cefazolin, Cefquinome, Ceftiofur. S2 1,2-dichlorobenzene, 16ß-hydroxystanozolol, 17a-nortestosterone, 17a-trenbolone, 17ß-estradiol, 17ß-trenbolone, 2 4 6-triaminopyrimidine-5-carbonitrile, 2 4-dimethylaniline, 2ß-hydroxy-N- deethyltiamulin, 2ß-hydroxytiamulin, 3-O-acetyltylosin, 4 4'-dinitrocarbanilide/Nicarbazin (500) , 4- demethylgriseofulvin, 4-methylaminoantipyrine, 5-hydroxyflunixin, 5-hydroxythiabendazole, 6- demethylgriseofulvin, 8a-hydroxymutilin, 8a-hydroxy-N-deethyltiamulin, 8a-hydroxytiamulin, Abamectin B1a, Acepromazine, Acetylisovaleryltylosin, Acyclovir, AHD, Aklomide,