Three Key Things in Health Care (July 21, 2020)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Wednesday, July 21, 2021 Count and Rate of Confirmed COVID-19 Cases and Tests Performed in MA by County, January 1, 2020 – July 20, 2021
Massachusetts Department of Public Health COVID-19 Dashboard – Wednesday, July 21, 2021 Count and Rate of Confirmed COVID-19 Cases and Tests Performed in MA by County, January 1, 2020 – July 20, 2021 Average Daily Relative Percent Case Count Total Tests Total Change in Total Case Incidence Rate Change in Total Positivity County (Last 14 (Last 14 Positive Percent Count per 100,000 Case Tests (Last 14 2 Days) days) Tests (Last Positivity (Last 14 days) Counts1 14 days) days) Barnstable County 14169 156 5.1 Higher 427494 7433 172 2.31% Higher Berkshire County 6598 22 1.2 Lower 422549 4907 28 0.57% Lower Bristol County 67311 270 3.4 Higher 1418783 20327 292 1.44% Higher Dukes and Nantucket Counties 2963 22 5.5 Higher 85351 1239 24 1.94% Higher Essex County 98320 241 2.2 Higher 2377043 30451 276 0.91% Higher Franklin County 2591 4 0.4 Higher 229265 3159 4 0.13% No Change Hampden County 52719 211 3.2 Higher 1245489 20072 242 1.21% Higher Hampshire County 9217 33 1.4 Higher 969709 10392 42 0.40% Higher Middlesex County 136114 526 2.3 Higher 6355716 90877 596 0.66% Higher Norfolk County 55331 243 2.4 Higher 2160679 32458 269 0.83% Higher Plymouth County 49548 179 2.4 Higher 1201437 18617 203 1.09% Higher Suffolk County 93688 404 3.6 Higher 4586292 57199 466 0.81% Higher Worcester County 77816 267 2.3 Higher 2525358 32252 308 0.95% Higher Unknown3 956 3 * * 324200 3415 3 * * State 667341 2581 2.6 Higher 24329365 332798 2925 0.88% Higher Data are current as of 11:59pm on 07/20/2021; 1Number of new cases occurring over the current two-week period (7/04/2021 – 7/17/2021) compared to the previous two-week period (6/27/2021 – 7/10/2021). -
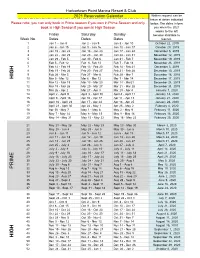
2021 Calandar
Harbortown Point Marina Resort & Club 2021 Reservation Calendar Written request can be taken at dates indicated Please note: you can only book in Prime season if you own in Prime Season and only below. The dates inform book in High Season if you own in High Season you when the 2021 weeks to the left Friday Saturday Sunday become abailable to Week No. Dates Dates Dates reserve. 1 Jan 1 - Jan 8 Jan 2 - Jan 9 Jan 3 - Jan 10 October 22, 2019 2 Jan 8 - Jan 15 Jan 9 - Jan 16 Jan 10 - Jan 17 October 29, 2019 3 Jan 15 - Jan 22 Jan 16 - Jan 23 Jan 17 - Jan 24 November 5, 2019 4 Jan 22 - Jan 29 Jan 23 - Jan 30 Jan 24 - Jan 31 November 12, 2019 5 Jan 29 - Feb 5 Jan 30 - Feb 6 Jan 31 - Feb 7 November 19, 2019 6 Feb 5 - Feb 12 Feb 6- Feb 13 Feb 7 - Feb 14 November 26, 2019 7 Feb 12 - Feb 19 Feb 13 - Feb 20 Feb 14 - Feb 21 December 3, 2019 8 Feb 19 - Feb 26 Feb 20 - Feb 27 Feb 21 - Feb 28 December 10, 2019 9 Feb 26 - Mar 5 Feb 27 - Mar 6 Feb 28 - Mar 7 December 18, 2018 HIGH 10 Mar 5 - Mar 12 Mar 6 - Mar 13 Mar 7 - Mar 14 December 17, 2019 11 Mar 12 - Mar 19 Mar 13 - Mar 20 Mar 14 - Mar21 December 24, 2019 12 Mar 19 - Mar 26 Mar 20 - Mar 27 Mar 21 - Mar 28 December 31, 2019 13 Mar 26 - Apr 2 Mar 27 - Apr 3 Mar 28 - Apr 4 January 7, 2020 14 April 2 - April 9 April 3 - April 10 April 4 - April 11 January 14, 2020 15 April 9 - April 16 Apr 10 - Apr 17 Apr 11 - Apr 18 January 21, 2020 16 April 16 - April 23 Apr 17 - Apr 24 Apr 18 - Apr 25 January 28, 2020 17 April 23 - April 30 Apr 24 - May 1 Apr 25 - May 2 February 4, 2020 18 Apr 30 - May 7 May 1 - May -

2021-2022 Custom & Standard Information Due Dates
2021-2022 CUSTOM & STANDARD INFORMATION DUE DATES Desired Cover All Desired Cover All Delivery Date Info. Due Text Due Delivery Date Info. Due Text Due May 31 No Deliveries No Deliveries July 19 April 12 May 10 June 1 February 23 March 23 July 20 April 13 May 11 June 2 February 24 March 24 July 21 April 14 May 12 June 3 February 25 March 25 July 22 April 15 May 13 June 4 February 26 March 26 July 23 April 16 May 14 June 7 March 1 March 29 July 26 April 19 May 17 June 8 March 2 March 30 July 27 April 20 May 18 June 9 March 3 March 31 July 28 April 21 May 19 June 10 March 4 April 1 July 29 April 22 May 20 June 11 March 5 April 2 July 30 April 23 May 21 June 14 March 8 April 5 August 2 April 26 May 24 June 15 March 9 April 6 August 3 April 27 May 25 June 16 March 10 April 7 August 4 April 28 May 26 June 17 March 11 April 8 August 5 April 29 May 27 June 18 March 12 April 9 August 6 April 30 May 28 June 21 March 15 April 12 August 9 May 3 May 28 June 22 March 16 April 13 August 10 May 4 June 1 June 23 March 17 April 14 August 11 May 5 June 2 June 24 March 18 April 15 August 12 May 6 June 3 June 25 March 19 April 16 August 13 May 7 June 4 June 28 March 22 April 19 August 16 May 10 June 7 June 29 March 23 April 20 August 17 May 11 June 8 June 30 March 24 April 21 August 18 May 12 June 9 July 1 March 25 April 22 August 19 May 13 June 10 July 2 March 26 April 23 August 20 May 14 June 11 July 5 March 29 April 26 August 23 May 17 June 14 July 6 March 30 April 27 August 24 May 18 June 15 July 7 March 31 April 28 August 25 May 19 June 16 July 8 April 1 April 29 August 26 May 20 June 17 July 9 April 2 April 30 August 27 May 21 June 18 July 12 April 5 May 3 August 30 May 24 June 21 July 13 April 6 May 4 August 31 May 25 June 22 July 14 April 7 May 5 September 1 May 26 June 23 July 15 April 8 May 6 September 2 May 27 June 24 July 16 April 9 May 7 September 3 May 28 June 25. -

PROCLAMATION 4771—JULY 2, 1980 94 STAT. 3775 Registration Under the Military Selective Service
PROCLAMATION 4771—JULY 2, 1980 94 STAT. 3775 Proclamation 4771 of July 2,1980 Registration Under the Military Selective Service Act By the President of the United States of America . A Proclamation Section 3 of the Military Selective Service Act, as amended (50 U.S.C. App. 453), provides that male citizens of the United States and other male per sons residing in the United States who are between the ages of 18 and 26, except those exempted by Sections 3 and 6(a) of the Military Selective Service Act, must present themselves for registration at such time or times 50 USC app. 456. and place or places, and in such manner as determined by the President. Section 6(k) provides that such exceptions shall not continue after the cause for the exemption ceases to exist. The Congress of the United States has made available the funds (H.J. Res. 521, approved by me on June 27, 1980), which are needed to initiate this Ante, p. 552. registration, beginning with those born on or after January 1, 1960. NOW, THEREFORE, I, JIMMY CARTER, President of the United States of America, by the authority vested in me by the Military Selective Service Act, as amended (50 U.S.C. App. 451 et seq.), do hereby proclaim as fol lows: 1-1. Persons to be Registered and Days of Registration. 1-101. Male citizens of the United States and other males residing in the United States, unless exempted by the MiHtary Selective Service Act, as amended, who were born on or after January 1, 1960, and who have at tained their eighteenth birthday, shall present themselves for registration in the manner and at the time and places as hereinafter provided. -

July 21, 2020
DELAWARE'S RESPONSE TO COVID-19 Press Briefing July 21, 2020 de.gov/coronavirus Delaware Cases of COVID-19 13,746 total cases 144,601 negative tests 525 total deaths 58 current hospitalizations 11 critical hospitalizations As of July 20, 2020 at 6:00 p.m. Delaware Cases of COVID-19 105.3 new cases per day (5-day average) 4.2 percent positive (5-day average) As of July 20, 2020 at 6:00 p.m. Delaware Cases of COVID-19 6,206 positive cases since first case confirmed on March 11, 2020 As of July 20, 2020 at 6:00 p.m. Delaware Cases of COVID-19 2,017 positive cases since first case confirmed on March 11, 2020 As of July 20, 2020 at 6:00 p.m. Delaware Cases of COVID-19 5,365 positive cases since first case confirmed on March 11, 2020 As of July 20, 2020 at 6:00 p.m. Total Cases by Age As of July 20, 2020 at 6:00 p.m. Rate of Cases by Age As of July 20, 2020 at 6:00 p.m. COVID-19 Data Dashboard Delaware's Personal Protective Equipment (PPE) Inventory Status View more data: de.gov/healthycommunity COVID-19 Data Dashboard View more data: de.gov/healthycommunity COVID-19 Data Dashboard 90-day View View more data: de.gov/healthycommunity Infection Rate: All Available Data Lives Lost TESTING POSITIVITY: Which U.S. States Meet WHO Recommended Testing Criteria? DE MD PA Data as of Tuesday, July 21, 2020 at 3:00 AM EDT Source: Johns Hopkins University Coronavirus Resource Center (coronavirus.jhu.edu/testing/testing-positivity) Schools: Planning Scenarios Schools provide more than education: • Meals • Mental health services • Socialization • Child care • Additional supports We are assigning an expert liaison to advise each school district on health decisions around reopening during COVID-19. -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -

COVID-19 Dashboard - Tuesday, July 21, 2020 Dashboard of Public Health Indicators
7/21/2020 Duplicate of Public Health Indicators Massachusetts Department of Public Health COVID-19 Dashboard - Tuesday, July 21, 2020 Dashboard of Public Health Indicators Newly Reported Total Confirmed Newly Reported Total Deaths Confirmed Cases Cases Deaths among among Confirmed Today Confirmed Today Cases 165 107,221 17 8,231 New Individuals Total Individuals Below is the status as of June 5, 2020: Tested by Tested by Molecular Tests Molecular Tests Measure Status 7,821 1,052,369 COVID-19 positive test rate ⚫ Number of individuals who died from COVID-19 ⚫ Number of patients with COVID-19 in hospitals ⚫ Total Molecular Legend Tests Healthcare system readiness ⚫ Administered Testing capacity ⚫ 1,353,802 Contact tracing capabilities ⚫ Please note: The front page of the dashboard has been reformatted. Probable case and death information can be found on page 21. Antibody tests (individual and total numbers) can be found on page 7. For more information on the COVID-19 case definition, see https://cdn.ymaws.com/www.cste.org/resource/resmgr/2020ps/Interim-20-ID-01_COVID-19.pdf 1 1/1 7/21/2020 Public Health Indicators2 Massachusetts Department of Public Health COVID-19 Dashboard - Tuesday, July 21, 2020 Percent Change Since Dashboard of Public Health Indicators April 15th 7 Day Weighted 2.0% 2.0% 2.0% 1.9% 1.9% 1.9% 1.9% Average of Positive 1.9% 2.0% 1.9% 1.9% 1.8% 1.9% 2.0% 1.8% 1.8% Molecular Test Rate 1.8% 1.8% 1.9% 1.9% 1.7% 1.7% 1.7% 1.8% 1.8% 1.7% 1.7% 1.7% 1.7% 1.7% 1.8% -94 % 1.6% 1.7% June June June June June June June June June June -
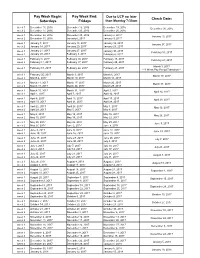
Pay Week Begin: Saturdays Pay Week End: Fridays Check Date
Pay Week Begin: Pay Week End: Due to UCP no later Check Date: Saturdays Fridays than Monday 7:30am week 1 December 10, 2016 December 16, 2016 December 19, 2016 December 30, 2016 week 2 December 17, 2016 December 23, 2016 December 26, 2016 week 1 December 24, 2016 December 30, 2016 January 2, 2017 January 13, 2017 week 2 December 31, 2016 January 6, 2017 January 9, 2017 week 1 January 7, 2017 January 13, 2017 January 16, 2017 January 27, 2017 week 2 January 14, 2017 January 20, 2017 January 23, 2017 January 21, 2017 January 27, 2017 week 1 January 30, 2017 February 10, 2017 week 2 January 28, 2017 February 3, 2017 February 6, 2017 week 1 February 4, 2017 February 10, 2017 February 13, 2017 February 24, 2017 week 2 February 11, 2017 February 17, 2017 February 20, 2017 March 3, 2017 week 1 February 18, 2017 February 24, 2017 February 27, 2017 ***1 Week Pay Period Transition*** week 1 February 25, 2017 March 3, 2017 March 6, 2017 March 17, 2017 week 2 March 4, 2017 March 10, 2017 March 13, 2017 week 1 March 11, 2017 March 17, 2017 March 20, 2017 March 31, 2017 week 2 March 18, 2017 March 24, 2017 March 27, 2017 week 1 March 25, 2017 March 31, 2017 April 3, 2017 April 14, 2017 week 2 April 1, 2017 April 7, 2017 April 10, 2017 week 1 April 8, 2017 April 14, 2017 April 17, 2017 April 28, 2017 week 2 April 15, 2017 April 21, 2017 April 24, 2017 week 1 April 22, 2017 April 28, 2017 May 1, 2017 May 12, 2017 week 2 April 29, 2017 May 5, 2017 May 8, 2017 week 1 May 6, 2017 May 12, 2017 May 15, 2017 May 26, 2017 week 2 May 13, 2017 May 19, 2017 May -

Operative Dates for Legislative Bills Enacted
APPENDIX CROSS REFERENCE TABLE Legislative Bills, One Hundred Fourth Legislature Second Session, 2016 Showing the date each act went into effect. Convened January 6, 2016, and adjourned April 20, 2016. LB No. Effective Date LB No. Effective Date 19 July 21, 2016 474 July 21, 2016 47 July 1, 2017 474 A July 21, 2016 (operative date) 505 January 1, 2017 53 July 21, 2016 (operative date) 83 July 21, 2016 505 A July 21, 2016 131 July 21, 2016 567 July 21, 2016 136 February 25, 2016 665 July 21, 2016 176 July 21, 2016 666 July 21, 2016 190 July 21, 2016 667 July 21, 2016 221 July 21, 2016 676 March 10, 2016 235 July 21, 2016 677 July 21, 2016 270 July 1, 2016 678 October 1, 2016 (operative date) (operative date) 275 July 21, 2016 679 July 21, 2016 285 July 21, 2016 680 April 7, 2016 295 July 21, 2016 680 A April 7, 2016 311 March 4, 2016 683 January 1, 2017 328 July 21, 2016 (operative date) 378 July 21, 2016 684 July 21, 2016 400 July 21, 2016 686 July 21, 2016 400 A July 21, 2016 694 July 21, 2016 447 Sections 17 and 52 of this 695 July 21, 2016 act become operative on 698 Sections 1, 2, 3, 4, 5, 6, 7, July 1, 2016. Sections 31 8, 9, 10, 11, 12, 13, 14, 17, and 53 of this act become 18, 19, 20, 21, 22, 23, 24, operative on September 1, 25, 26, 27, 28, 29, 30, 31, 2016. -

Julian Date Cheat Sheet for Regular Years
Date Code Cheat Sheet For Regular Years Day of Year Calendar Date 1 January 1 2 January 2 3 January 3 4 January 4 5 January 5 6 January 6 7 January 7 8 January 8 9 January 9 10 January 10 11 January 11 12 January 12 13 January 13 14 January 14 15 January 15 16 January 16 17 January 17 18 January 18 19 January 19 20 January 20 21 January 21 22 January 22 23 January 23 24 January 24 25 January 25 26 January 26 27 January 27 28 January 28 29 January 29 30 January 30 31 January 31 32 February 1 33 February 2 34 February 3 35 February 4 36 February 5 37 February 6 38 February 7 39 February 8 40 February 9 41 February 10 42 February 11 43 February 12 44 February 13 45 February 14 46 February 15 47 February 16 48 February 17 49 February 18 50 February 19 51 February 20 52 February 21 53 February 22 54 February 23 55 February 24 56 February 25 57 February 26 58 February 27 59 February 28 60 March 1 61 March 2 62 March 3 63 March 4 64 March 5 65 March 6 66 March 7 67 March 8 68 March 9 69 March 10 70 March 11 71 March 12 72 March 13 73 March 14 74 March 15 75 March 16 76 March 17 77 March 18 78 March 19 79 March 20 80 March 21 81 March 22 82 March 23 83 March 24 84 March 25 85 March 26 86 March 27 87 March 28 88 March 29 89 March 30 90 March 31 91 April 1 92 April 2 93 April 3 94 April 4 95 April 5 96 April 6 97 April 7 98 April 8 99 April 9 100 April 10 101 April 11 102 April 12 103 April 13 104 April 14 105 April 15 106 April 16 107 April 17 108 April 18 109 April 19 110 April 20 111 April 21 112 April 22 113 April 23 114 April 24 115 April -
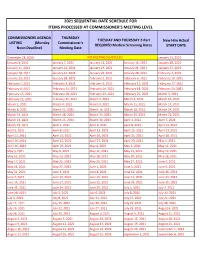
2021 Sequential Date List
2021 SEQUENTIAL DATE SCHEDULE FOR ITEMS PROCESSED AT COMMISSIONER'S MEETING LEVEL COMMISSIONERS AGENDA THURSDAY TUESDAY AND THURSDAY 2-Part New Hire Actual LISTING (Monday Commissioner's REQUIRED Medical Screening Dates START DATE Noon Deadline) Meeting Date December 28, 2020 NO MEETING SCHEDULED January 13, 2021 January 4, 2021 January 7, 2021 January 12, 2021 January 14, 2021 January 20, 2021 January 11, 2021 January 14, 2021 January 19, 2021 January 21, 2021 January 27, 2021 January 18, 2021 January 21, 2021 January 26, 2021 January 28, 2021 February 3, 2021 January 25, 2021 January 28, 2021 February 2, 2021 February 4, 2021 February 10, 2021 February 1, 2021 February 4, 2021 February 9, 2021 February 11, 2021 February 17, 2021 February 8, 2021 February 11, 2021 February 16, 2021 February 18, 2021 February 24, 2021 February 15, 2021 February 18, 2021 February 23, 2021 February 25, 2021 March 3, 2021 February 22, 2021 February 25, 2021 March 2, 2021 March 4, 2021 March 10, 2021 March 1, 2021 March 4, 2021 March 9, 2021 March 11, 2021 March 17, 2021 March 8, 2021 March 11, 2021 March 16, 2021 March 18, 2021 March 24, 2021 March 15, 2021 March 18, 2021 March 23, 2021 March 25, 2021 March 31, 2021 March 22, 2021 March 25, 2021 March 30, 2021 April 1, 2021 April 7, 2021 March 29, 2021 April 1, 2021 April 6, 2021 April 8, 2021 April 14, 2021 April 5, 2021 April 8, 2021 April 13, 2021 April 15, 2021 April 21, 2021 April 12, 2021 April 15, 2021 April 20, 2021 April 22, 2021 April 28, 2021 April 19, 2021 April 22, 2021 April 27, 2021 April -

Pay Date Calendar
Pay Date Information Select the pay period start date that coincides with your first day of employment. Pay Period Pay Period Begins (Sunday) Pay Period Ends (Saturday) Official Pay Date (Thursday)* 1 January 10, 2016 January 23, 2016 February 4, 2016 2 January 24, 2016 February 6, 2016 February 18, 2016 3 February 7, 2016 February 20, 2016 March 3, 2016 4 February 21, 2016 March 5, 2016 March 17, 2016 5 March 6, 2016 March 19, 2016 March 31, 2016 6 March 20, 2016 April 2, 2016 April 14, 2016 7 April 3, 2016 April 16, 2016 April 28, 2016 8 April 17, 2016 April 30, 2016 May 12, 2016 9 May 1, 2016 May 14, 2016 May 26, 2016 10 May 15, 2016 May 28, 2016 June 9, 2016 11 May 29, 2016 June 11, 2016 June 23, 2016 12 June 12, 2016 June 25, 2016 July 7, 2016 13 June 26, 2016 July 9, 2016 July 21, 2016 14 July 10, 2016 July 23, 2016 August 4, 2016 15 July 24, 2016 August 6, 2016 August 18, 2016 16 August 7, 2016 August 20, 2016 September 1, 2016 17 August 21, 2016 September 3, 2016 September 15, 2016 18 September 4, 2016 September 17, 2016 September 29, 2016 19 September 18, 2016 October 1, 2016 October 13, 2016 20 October 2, 2016 October 15, 2016 October 27, 2016 21 October 16, 2016 October 29, 2016 November 10, 2016 22 October 30, 2016 November 12, 2016 November 24, 2016 23 November 13, 2016 November 26, 2016 December 8, 2016 24 November 27, 2016 December 10, 2016 December 22, 2016 25 December 11, 2016 December 24, 2016 January 5, 2017 26 December 25, 2016 January 7, 2017 January 19, 2017 1 January 8, 2017 January 21, 2017 February 2, 2017 2 January